Epigenetic reprogramming by TET enzymes impacts co-transcriptional R-loops
- PMID: 35191837
- PMCID: PMC8896830
- DOI: 10.7554/eLife.69476
Epigenetic reprogramming by TET enzymes impacts co-transcriptional R-loops
Abstract
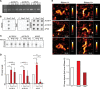
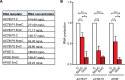
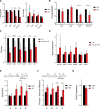
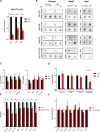
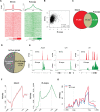
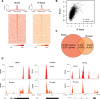
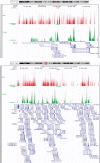
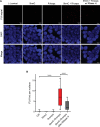
DNA oxidation by ten-eleven translocation (TET) family enzymes is essential for epigenetic reprogramming. The conversion of 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) initiates developmental and cell-type-specific transcriptional programs through mechanisms that include changes in the chromatin structure. Here, we show that the presence of 5hmC in the transcribed gene promotes the annealing of the nascent RNA to the template DNA strand, leading to the formation of an R-loop. Depletion of TET enzymes reduced global R-loops in the absence of gene expression changes, whereas CRISPR-mediated tethering of TET to an active gene promoted the formation of R-loops. The genome-wide distribution of 5hmC and R-loops shows a positive correlation in mouse and human stem cells and overlap in half of all active genes. Moreover, R-loop resolution leads to differential expression of a subset of genes that are involved in crucial events during stem cell proliferation. Altogether, our data reveal that epigenetic reprogramming via TET activity promotes co-transcriptional R-loop formation, disclosing new mechanisms of gene expression regulation.
Keywords: DNA hydroxymethylation; R-loops; TET enzymes; chromosomes; gene expression; human; mouse; stem cells.
© 2022, Sabino et al.
Conflict of interest statement
JS, Md, PA, AF, PC, MD, NS, CA, AG, Sd No competing interests declared
Figures


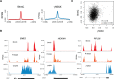
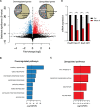
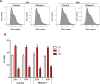
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

