TNF-α inhibitor tanfanercept (HBM9036) improves signs and symptoms of dry eye in a phase 2 trial in the controlled adverse environment in China
- PMID: 35192105
- PMCID: PMC9314282
- DOI: 10.1007/s10792-022-02245-1
TNF-α inhibitor tanfanercept (HBM9036) improves signs and symptoms of dry eye in a phase 2 trial in the controlled adverse environment in China
Abstract
Purpose: This study evaluated the clinical safety and efficacy of tanfanercept (HBM9036) ophthalmic solution as a novel treatment for dry eye disease (DED) in a controlled adverse environment (CAE) study conducted in China.
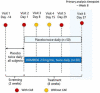
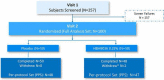
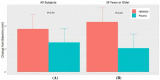
Methods: In a single-center, double-masked, randomized, placebo-controlled study, 100 patients received 0.25% tanfanercept, or placebo, twice daily for eight weeks. A mobile international CAE® DE Model was used for patient selection with a standardized challenge endpoint. Primary efficacy endpoint was fluorescein inferior corneal staining score (ICSS) pre- to post-CAE challenge from baseline. Secondary endpoints included Schirmer's Tear Test, Tear-Film Break-Up Time, Ocular Discomfort Score, Ora Calibra® Ocular Discomfort and 4-Symptom Questionnaire, total corneal staining score (TCSS), and drop comfort. Signs and symptoms were assessed both pre- and post-CAE to evaluate the efficacy of tanfanercept on both environmental and CAE endpoints.
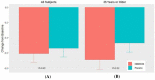
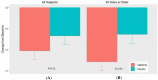
Results: The tanfanercept treatment group showed improvement in ICSS pre- to post-CAE change from baseline scores when compared to placebo (- 0.61 ± 0.11 and - 0.54 ± 0.11, respectively; mean difference = 0.07, p = 0.65). TCSS pre-post-CAE change from baseline scores was also in favor of active when compared to placebo (- 1.03 ± 0.21 and - 0.67 ± 0.21, respectively; mean difference = 0.37, p = 0.23). Schirmer's score improvement was demonstrated in favor of active (1.87 ± 0.62 mm) as compared to placebo (1.28 ± 0.62 mm; mean difference = 0.59 mm, p = 0.50). Change from baseline in mean Tear-Film Break-up Time favored active treatment over placebo (mean difference = 1.21 s, p = 0.45). Notably, the tanfanercept showed more obvious benefits for each DED sign in a subgroup of subjects ≥ 35 years of age. Tanfanercept was well tolerated with no serious adverse events occurring during the study.
Conclusion: Tanfanercept demonstrated improvements in favor of active as compared to placebo in the signs of DED, being safe and well tolerated. These data support further evaluation of tanfanercept for the treatment of DED in China.
Trial registration: This study was retrospectively registered at ClinicalTrials.gov (NCT04092907) on September 17, 2019.
Keywords: Controlled adverse environment; Dry eye disease; TNF-TNFR1 inhibitor; Tanfanercept.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures

Similar articles
-
A phase 2 randomized, double-masked, placebo-controlled study of novel nonsystemic kinase inhibitor TOP1630 for the treatment of dry eye disease.Clin Ophthalmol. 2019 Feb 12;13:261-275. doi: 10.2147/OPTH.S189039. eCollection 2019. Clin Ophthalmol. 2019. PMID: 30858682 Free PMC article.
-
A Phase 2 Trial to Test Safety and Efficacy of ST-100, a Unique Collagen Mimetic Peptide Ophthalmic Solution for Dry Eye Disease.Ophthalmol Sci. 2023 Dec 12;4(3):100451. doi: 10.1016/j.xops.2023.100451. eCollection 2024 May-Jun. Ophthalmol Sci. 2023. PMID: 38317866 Free PMC article.
-
Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study.Ophthalmology. 2012 Dec;119(12):2471-8. doi: 10.1016/j.ophtha.2012.06.052. Epub 2012 Sep 23. Ophthalmology. 2012. PMID: 23009892 Clinical Trial.
-
Quintessence of currently approved and upcoming treatments for dry eye disease.Graefes Arch Clin Exp Ophthalmol. 2025 Feb;263(2):269-278. doi: 10.1007/s00417-024-06587-7. Epub 2024 Aug 31. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 39215848 Review.
-
A review of the first anti-evaporative prescription treatment for dry eye disease: perfluorohexyloctane ophthalmic solution.Am J Manag Care. 2023 Nov;29(14 Suppl):S251-S259. doi: 10.37765/ajmc.2023.89464. Am J Manag Care. 2023. PMID: 37930231 Review.
Cited by
-
Tolerability of Current Treatments for Dry Eye Disease: A Review of Approved and Investigational Therapies.Clin Ophthalmol. 2024 Aug 16;18:2283-2302. doi: 10.2147/OPTH.S465143. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39165367 Free PMC article. Review.
-
Recent United States Developments in the Pharmacological Treatment of Dry Eye Disease.Drugs. 2024 May;84(5):549-563. doi: 10.1007/s40265-024-02031-6. Epub 2024 Apr 23. Drugs. 2024. PMID: 38652355 Free PMC article. Review.
-
Role of topical and systemic immunosuppression in aqueous-deficient dry eye disease.Indian J Ophthalmol. 2023 Apr;71(4):1176-1189. doi: 10.4103/IJO.IJO_2818_22. Indian J Ophthalmol. 2023. PMID: 37026249 Free PMC article. Review.
-
The Exacerbating Effects of the Tumor Necrosis Factor in Cardiovascular Stenosis: Intimal Hyperplasia.Cancers (Basel). 2024 Apr 8;16(7):1435. doi: 10.3390/cancers16071435. Cancers (Basel). 2024. PMID: 38611112 Free PMC article. Review.
-
Polymorphisms in Lymphotoxin-Alpha as the "Missing Link" in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review.Int J Mol Sci. 2023 Feb 20;24(4):4236. doi: 10.3390/ijms24044236. Int J Mol Sci. 2023. PMID: 36835647 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

