Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression
- PMID: 35192546
- PMCID: PMC8970674
- DOI: 10.1172/JCI153920
Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression
Abstract
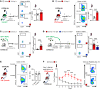
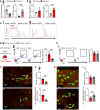
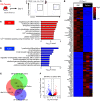
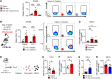
Platelets have a wide range of functions including critical roles in hemostasis, thrombosis, and immunity. We hypothesized that during acute inflammation, such as in life-threatening sepsis, there are fundamental changes in the sites of platelet production and phenotypes of resultant platelets. Here, we showed during sepsis that the spleen was a major site of megakaryopoiesis and platelet production. Sepsis provoked an adrenergic-dependent mobilization of megakaryocyte-erythrocyte progenitors (MEPs) from the bone marrow to the spleen, where IL-3 induced their differentiation into megakaryocytes (MKs). In the spleen, immune-skewed MKs produced a CD40 ligandhi platelet population with potent immunomodulatory functions. Transfusions of post-sepsis platelets enriched from splenic production enhanced immune responses and reduced overall mortality in sepsis-challenged animals. These findings identify a spleen-derived protective platelet population that may be broadly immunomodulatory in acute inflammatory states such as sepsis.
Keywords: Hematology; Hematopoietic stem cells; Innate immunity; Platelets; Stem cells.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

