Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England
- PMID: 35192597
- PMCID: PMC8863280
- DOI: 10.1371/journal.pmed.1003926
Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England
Abstract
Background: Thromboses in unusual locations after the Coronavirus Disease 2019 (COVID-19) vaccine ChAdOx1-S have been reported, although their frequency with vaccines of different types is uncertain at a population level. The aim of this study was to estimate the population-level risks of hospitalised thrombocytopenia and major arterial and venous thromboses after COVID-19 vaccination.
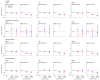
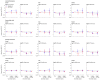
Methods and findings: In this whole-population cohort study, we analysed linked electronic health records from adults living in England, from 8 December 2020 to 18 March 2021. We estimated incidence rates and hazard ratios (HRs) for major arterial, venous, and thrombocytopenic outcomes 1 to 28 and >28 days after first vaccination dose for ChAdOx1-S and BNT162b2 vaccines. Analyses were performed separately for ages <70 and ≥70 years and adjusted for age, age2, sex, ethnicity, and deprivation. We also prespecified adjustment for anticoagulant medication, combined oral contraceptive medication, hormone replacement therapy medication, history of pulmonary embolism or deep vein thrombosis, and history of coronavirus infection in analyses of venous thrombosis; and diabetes, hypertension, smoking, antiplatelet medication, blood pressure lowering medication, lipid lowering medication, anticoagulant medication, history of stroke, and history of myocardial infarction in analyses of arterial thromboses. We selected further covariates with backward selection. Of 46 million adults, 23 million (51%) were women; 39 million (84%) were <70; and 3.7 million (8.1%) Asian or Asian British, 1.6 million (3.5%) Black or Black British, 36 million (79%) White, 0.7 million (1.5%) mixed ethnicity, and 1.5 million (3.2%) were of another ethnicity. Approximately 21 million (46%) adults had their first vaccination between 8 December 2020 and 18 March 2021. The crude incidence rates (per 100,000 person-years) of all venous events were as follows: prevaccination, 140 [95% confidence interval (CI): 138 to 142]; ≤28 days post-ChAdOx1-S, 294 (281 to 307); >28 days post-ChAdOx1-S, 359 (338 to 382), ≤28 days post-BNT162b2-S, 241 (229 to 253); >28 days post-BNT162b2-S 277 (263 to 291). The crude incidence rates (per 100,000 person-years) of all arterial events were as follows: prevaccination, 546 (95% CI: 541 to 555); ≤28 days post-ChAdOx1-S, 1,211 (1,185 to 1,237); >28 days post-ChAdOx1-S, 1678 (1,630 to 1,726), ≤28 days post-BNT162b2-S, 1,242 (1,214 to 1,269); >28 days post-BNT162b2-S, 1,539 (1,507 to 1,572). Adjusted HRs (aHRs) 1 to 28 days after ChAdOx1-S, compared with unvaccinated rates, at ages <70 and ≥70 years, respectively, were 0.97 (95% CI: 0.90 to 1.05) and 0.58 (0.53 to 0.63) for venous thromboses, and 0.90 (0.86 to 0.95) and 0.76 (0.73 to 0.79) for arterial thromboses. Corresponding aHRs for BNT162b2 were 0.81 (0.74 to 0.88) and 0.57 (0.53 to 0.62) for venous thromboses, and 0.94 (0.90 to 0.99) and 0.72 (0.70 to 0.75) for arterial thromboses. aHRs for thrombotic events were higher at younger ages for venous thromboses after ChAdOx1-S, and for arterial thromboses after both vaccines. Rates of intracranial venous thrombosis (ICVT) and of thrombocytopenia in adults aged <70 years were higher 1 to 28 days after ChAdOx1-S (aHRs 2.27, 95% CI: 1.33 to 3.88 and 1.71, 1.35 to 2.16, respectively), but not after BNT162b2 (0.59, 0.24 to 1.45 and 1.00, 0.75 to 1.34) compared with unvaccinated. The corresponding absolute excess risks of ICVT 1 to 28 days after ChAdOx1-S were 0.9 to 3 per million, varying by age and sex. The main limitations of the study are as follows: (i) it relies on the accuracy of coded healthcare data to identify exposures, covariates, and outcomes; (ii) the use of primary reason for hospital admission to measure outcome, which improves the positive predictive value but may lead to an underestimation of incidence; and (iii) potential unmeasured confounding.
Conclusions: In this study, we observed increases in rates of ICVT and thrombocytopenia after ChAdOx1-S vaccination in adults aged <70 years that were small compared with its effect in reducing COVID-19 morbidity and mortality, although more precise estimates for adults aged <40 years are needed. For people aged ≥70 years, rates of arterial or venous thrombotic events were generally lower after either vaccine compared with unvaccinated, suggesting that either vaccine is suitable in this age group.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: WW has given expert testimony to UK courts. WW served on a advisory board for Bayer. CS is Director of the BHF Data Science Centre (at Health Data Research UK), which worked with NHS Digital to develop its Trusted Research Environment. CS leads the CVD-COVID-UK consortium which has enabled access to the linked population health data that enabled this study. No other authors declared conflicts of interest.
Figures

Comment in
-
Vaccine equity: A fundamental imperative in the fight against COVID-19.PLoS Med. 2022 Feb 22;19(2):e1003948. doi: 10.1371/journal.pmed.1003948. eCollection 2022 Feb. PLoS Med. 2022. PMID: 35192620 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- MC/UU/00011/4/MRC_/Medical Research Council/United Kingdom
- C18081/A31373/CRUK_/Cancer Research UK/United Kingdom
- RG/13/13/30194/DH_/Department of Health/United Kingdom
- SP/19/3/34678/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_20030/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_PC_20059/MRC_/Medical Research Council/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- MC_PC_20051/MRC_/Medical Research Council/United Kingdom
- SP/18/3/33801/BHF_/British Heart Foundation/United Kingdom
- MC_PC_20058/MRC_/Medical Research Council/United Kingdom
- MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/4/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

