Allele imputation for the killer cell immunoglobulin-like receptor KIR3DL1/S1
- PMID: 35192601
- PMCID: PMC8896733
- DOI: 10.1371/journal.pcbi.1009059
Allele imputation for the killer cell immunoglobulin-like receptor KIR3DL1/S1
Abstract
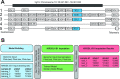
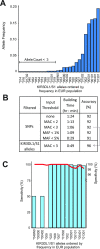
Highly polymorphic interaction of KIR3DL1 and KIR3DS1 with HLA class I ligands modulates the effector functions of natural killer (NK) cells and some T cells. This genetically determined diversity affects severity of infections, immune-mediated diseases, and some cancers, and impacts the course of immunotherapies, including transplantation. KIR3DL1 is an inhibitory receptor, and KIR3DS1 is an activating receptor encoded by the KIR3DL1/S1 gene that has more than 200 diverse and divergent alleles. Determination of KIR3DL1/S1 genotypes for medical application is hampered by complex sequence and structural variation, requiring targeted approaches to generate and analyze high-resolution allele data. To overcome these obstacles, we developed and optimized a model for imputing KIR3DL1/S1 alleles at high-resolution from whole-genome SNP data. We designed the model to represent a substantial component of human genetic diversity. Our Global imputation model is effective at genotyping KIR3DL1/S1 alleles with an accuracy ranging from 88% in Africans to 97% in East Asians, with mean specificity of 99% and sensitivity of 95% for alleles >1% frequency. We used the established algorithm of the HIBAG program, in a modification named Pulling Out Natural killer cell Genomics (PONG). Because HIBAG was designed to impute HLA alleles also from whole-genome SNP data, PONG allows combinatorial diversity of KIR3DL1/S1 with HLA-A and -B to be analyzed using complementary techniques on a single data source. The use of PONG thus negates the need for targeted sequencing data in very large-scale association studies where such methods might not be tractable.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Dr. Stephen Leslie is a partner with Peptide Groove LLP. All other authors have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

