Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma
- PMID: 35192934
- PMCID: PMC9171149
- DOI: 10.1016/j.ymthe.2022.02.021
Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma
Abstract
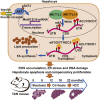
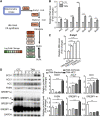
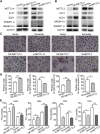
Type 2 diabetes mellitus (DM2) is associated closely with non-alcoholic fatty liver disease (NAFLD) by affecting lipid metabolism, which may lead to non-alcoholic steatohepatitis (NASH), fibrosis, and hepatocellular carcinoma (HCC). N6-methyladenosine (m6A) RNA methylation is an important epigenetic regulation for gene expression and is related to HCC development. We developed a new NAFLD model oriented from DM2 mouse, which spontaneously progressed to histological features of NASH, fibrosis, and HCC with high incidence. By RNA sequencing, protein expression and methylated RNA immunoprecipitation (MeRIP)-qPCR analysis, we found that enhanced expression of ACLY and SCD1 in this NAFLD model and human HCC samples was due to excessive m6A modification, but not elevation of mature SREBP1. Moreover, targeting METTL3/14 in vitro increases protein level of ACLY and SCD1 as well as triglyceride and cholesterol production and accumulation of lipid droplets. m6A sequencing analysis revealed that overexpressed METTL14 binds to mRNA of ACLY and SCD1 and alters their expression pattern. Our findings demonstrate a new NAFLD mouse model that provides a study platform for DM2-related NAFLD and reveals a unique epitranscriptional regulating mechanism for lipid metabolism via m6A-modified protein expression of ACLY and SCD1.
Keywords: ACLY; METTL14; SCD1; lipid metabolism; type 2 diabetes mellitus.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Ruggieri A., Barbati C., Malorni W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int. J. Cancer. 2010;127:499–504. - PubMed
-
- Huang T.D., Behary J., Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2020;50:1038–1047. - PubMed
-
- Buechler C., Aslanidis C. Role of lipids in pathophysiology, diagnosis and therapy of hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158658. - PubMed
-
- Ahmed M.H., Byrne C.D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD) Drug Discov. Today. 2007;12:740–747. - PubMed
-
- Li L., Che L., Tharp K.M., Park H.M., Pilo M.G., Cao D., Cigliano A., Latte G., Xu Z., Ribback S., Dombrowski F., Evert M., Gores G.J., Stahl A., Calvisi D.F., Chen X. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology. 2016;63:1900–1913. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

