Effects of Atrial Fibrillation on the Human Ventricle
- PMID: 35193397
- PMCID: PMC8963444
- DOI: 10.1161/CIRCRESAHA.121.319718
Effects of Atrial Fibrillation on the Human Ventricle
Abstract
Rationale: Atrial fibrillation (AF) and heart failure often coexist, but their interaction is poorly understood. Clinical data indicate that the arrhythmic component of AF may contribute to left ventricular (LV) dysfunction.
Objective: This study investigates the effects and molecular mechanisms of AF on the human LV.
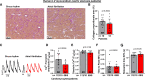
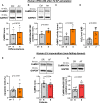
Methods and results: Ventricular myocardium from patients with aortic stenosis and preserved LV function with sinus rhythm or rate-controlled AF was studied. LV myocardium from patients with sinus rhythm and patients with AF showed no differences in fibrosis. In functional studies, systolic Ca2+ transient amplitude of LV cardiomyocytes was reduced in patients with AF, while diastolic Ca2+ levels and Ca2+ transient kinetics were not statistically different. These results were confirmed in LV cardiomyocytes from nonfailing donors with sinus rhythm or AF. Moreover, normofrequent AF was simulated in vitro using arrhythmic or rhythmic pacing (both at 60 bpm). After 24 hours of AF-simulation, human LV cardiomyocytes from nonfailing donors showed an impaired Ca2+ transient amplitude. For a standardized investigation of AF-simulation, human iPSC-cardiomyocytes were tested. Seven days of AF-simulation caused reduced systolic Ca2+ transient amplitude and sarcoplasmic reticulum Ca2+ load likely because of an increased diastolic sarcoplasmic reticulum Ca2+ leak. Moreover, cytosolic Na+ concentration was elevated and action potential duration was prolonged after AF-simulation. We detected an increased late Na+ current as a potential trigger for the detrimentally altered Ca2+/Na+-interplay. Mechanistically, reactive oxygen species were higher in the LV of patients with AF. CaMKII (Ca2+/calmodulin-dependent protein kinase IIδc) was found to be more oxidized at Met281/282 in the LV of patients with AF leading to an increased CaMKII activity and consequent increased RyR2 phosphorylation. CaMKII inhibition and ROS scavenging ameliorated impaired systolic Ca2+ handling after AF-simulation.
Conclusions: AF causes distinct functional and molecular remodeling of the human LV. This translational study provides the first mechanistic characterization and the potential negative impact of AF in the absence of tachycardia on the human ventricle.
Keywords: atrial fibrillation; calcium-calmodulin-dependent protein kinase type 2; excitation contraction coupling; heart failure; oxidative stress.
Figures





Comment in
-
Atrial fibrillation induces functional remodelling of the left ventricle.Nat Rev Cardiol. 2022 May;19(5):285. doi: 10.1038/s41569-022-00689-7. Nat Rev Cardiol. 2022. PMID: 35260796 No abstract available.
-
Heart Failure and Atrial Fibrillation-Chicken or Egg?Circ Res. 2022 Apr;130(7):1011-1013. doi: 10.1161/CIRCRESAHA.122.320930. Epub 2022 Mar 31. Circ Res. 2022. PMID: 35357896 No abstract available.
References
-
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946 - PubMed
-
- Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306 - PubMed
-
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E - PubMed
-
- Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. ; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

