A cell-based ELISA as surrogate of virus neutralization assay for RBD SARS-CoV-2 specific antibodies
- PMID: 35193792
- PMCID: PMC8856731
- DOI: 10.1016/j.vaccine.2022.02.044
A cell-based ELISA as surrogate of virus neutralization assay for RBD SARS-CoV-2 specific antibodies
Abstract
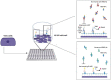
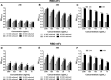
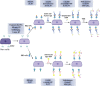
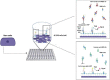
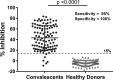
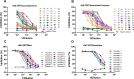
SARS-CoV-2, the cause of the COVID-19 pandemic, has provoked a global crisis and death of millions of people. Several serological assays to determine the quality of the immune response against SARS-CoV-2 and the efficacy of vaccines have been developed, among them the gold standard conventional virus neutralization assays. However, these tests are time consuming, require biosafety level 3 (BSL3), and are low throughput and expensive. This has motivated the development of alternative methods, including molecular inhibition assays. Herein, we present a safe cell-based ELISA-virus neutralization test (cbE-VNT) as a surrogate for the conventional viral neutralization assays that detects the inhibition of SARS-CoV-2 RBD binding to ACE2-bearing cells independently of species. Our test shows a very good correlation with the conventional and molecular neutralization assays and achieves 100% specificity and 95% sensitivity. cbE-VNT is cost-effective, fast and enables a large-scale serological evaluation that can be performed in a BSL2 laboratory, allowing its use in pre-clinical and clinical investigations.
Keywords: SARS-CoV-2; Vaccine; cVNT; cell-based ELISA; mVNT.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- https://www.statista.com/statistics/1087466/covid19-cases-recoveries-dea.... Number of coronavirus (COVID-19) cases, recoveries, and deaths worldwide as of November 23, 2021.
-
- https://www.who.int/publications/m/Item/draft-landscape-of-covid-19-cand.... Landscape of novel coronavirus candidate vaccines development worldwide. July 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

