Impact of the Sinopharm's BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE)
- PMID: 35193793
- PMCID: PMC8857641
- DOI: 10.1016/j.vaccine.2022.02.039
Impact of the Sinopharm's BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE)
Abstract
Background: This is a community-based, retrospective, observational study conducted to determine effectiveness of the BBIBP-CorV inactivated vaccine in the real-world setting against hospital admissions and death.
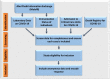
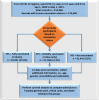
Study design: Study participants were selected from 214,940 PCR-positive cases of COVID-19 reported to the Department of Health, Abu Dhabi Emirate, United Arab Emirates (UAE) between September 01, 2020 and May 1, 2021. Of these, 176,640 individuals were included in the study who were aged ≥ 15 years with confirmed COVID-19 positive status who had records linked to their vaccination status. Those with incomplete or missing records were excluded (n = 38,300). Study participants were divided into three groups depending upon their vaccination status: fully vaccinated (two doses), partially vaccinated (single dose), and non-vaccinated. Study outcomes included COVID-19-related admissions to hospital general and critical care wards and death. Vaccine effectiveness for each outcome was based on the incidence density per 1000 person-years.
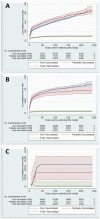
Results: The fully-, partially- and non-vaccinated groups included 62,931, 21,768 and 91,941 individuals, respectively. Based on the incidence rate ratios, the vaccine effectiveness in fully vaccinated individuals was 80%, 92%, and 97% in preventing COVID-19-related hospital admissions, critical care admissions, and death, respectively, when compared to the non-vaccinated group. No protection was observed for critical and non-critical care hospital admissions for the partially vaccinated group, while some protection against death was apparent, although statistically insignificant.
Conclusions: In a COVID-19 pandemic, use of the Sinopharm BBIBP-CorV inactivated vaccine is effective in preventing severe disease and death in a two-dose regimen. Lack of protection with the single dose may be explained by insufficient seroconversion and/or neutralizing antibody responses, behavioral factors (i.e., false sense of protection), and/or other biological factors (emergence of variants, possibility of reinfection, duration of vaccine protection, etc.).
Keywords: COVID-19; Inactivated viral vaccine; Real-world vaccine effectiveness study; SARS-CoV-2; Sinopharm BBIBP-CorV vaccine; United Arab Emirates (UAE).
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco.BMC Public Health. 2022 Aug 20;22(1):1584. doi: 10.1186/s12889-022-14016-9. BMC Public Health. 2022. PMID: 35987605 Free PMC article.
-
The incidence of COVID-19 infection following emergency use authorization of BBIBP-CORV inactivated vaccine in frontline workers in the United Arab Emirates.Sci Rep. 2022 Jan 11;12(1):490. doi: 10.1038/s41598-021-04244-1. Sci Rep. 2022. PMID: 35017530 Free PMC article.
-
Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID-19 in Abu Dhabi, United Arab Emirates.Nat Commun. 2022 Jun 9;13(1):3215. doi: 10.1038/s41467-022-30835-1. Nat Commun. 2022. PMID: 35680857 Free PMC article.
-
CoronaVac: A review of efficacy, safety, and immunogenicity of the inactivated vaccine against SARS-CoV-2.Hum Vaccin Immunother. 2022 Nov 30;18(6):2096970. doi: 10.1080/21645515.2022.2096970. Epub 2022 Jul 25. Hum Vaccin Immunother. 2022. PMID: 35878789 Free PMC article. Review.
-
A Review of COVID-19 Mass Testing in the United Arab Emirates.Front Public Health. 2021 May 12;9:661134. doi: 10.3389/fpubh.2021.661134. eCollection 2021. Front Public Health. 2021. PMID: 34055725 Free PMC article. Review.
Cited by
-
Adverse Effects of Sinopharm COVID-19 Vaccine among Vaccinated Medical Students and Health Care Workers.Vaccines (Basel). 2023 Jan 1;11(1):105. doi: 10.3390/vaccines11010105. Vaccines (Basel). 2023. PMID: 36679950 Free PMC article.
-
Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: Evidence from an outbreak in Yunnan, China, 2021.Vaccine. 2022 May 3;40(20):2869-2874. doi: 10.1016/j.vaccine.2022.03.067. Epub 2022 Apr 1. Vaccine. 2022. PMID: 35400561 Free PMC article.
-
Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Protection against SARS-CoV-2 Related Disease.Vaccines (Basel). 2022 Jun 9;10(6):920. doi: 10.3390/vaccines10060920. Vaccines (Basel). 2022. PMID: 35746530 Free PMC article.
-
Efficacy and effectiveness of inactivated vaccines against symptomatic COVID-19, severe COVID-19, and COVID-19 clinical outcomes in the general population: a systematic review and meta-analysis.Lancet Reg Health West Pac. 2023 May 20;37:100788. doi: 10.1016/j.lanwpc.2023.100788. Online ahead of print. Lancet Reg Health West Pac. 2023. PMID: 37360863 Free PMC article.
-
Vitamin D as an Adjuvant Immune Enhancer to SARS-Cov-2 Vaccine.Curr Microbiol. 2025 Feb 7;82(3):122. doi: 10.1007/s00284-025-04095-3. Curr Microbiol. 2025. PMID: 39918738
References
-
- COVID-19 Dashboard at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed Feb. 13, 2022.
-
- COVID-19 vaccine tracker and landscape. World Health Organization. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... Accessed Feb. 13, 2022.
-
- COVID-19 Vaccine Tracker. https://covid19.trackvaccines.org/. Accessed Feb. 13, 2022.
-
- World Health Organization (WHO) Guidelines. https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-v.... Accessed Oct. 18, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

