Loss of YTHDF1 in gastric tumors restores sensitivity to antitumor immunity by recruiting mature dendritic cells
- PMID: 35193930
- PMCID: PMC9066370
- DOI: 10.1136/jitc-2021-003663
Loss of YTHDF1 in gastric tumors restores sensitivity to antitumor immunity by recruiting mature dendritic cells
Abstract
Background: Gastric cancer (GC) is one of the most common cancer worldwide. We analyzed the expression of m6A regulatory genes in GC cohorts and revealed that YTHDF1 was uniquely upregulated in GC as compared with adjacent normal tissues. In this study, we analyzed the role of YTHDF1 in GC cells and modulation of the tumor immune microenvironment.
Methods: Three GC cohorts (cohort 1, n=101; cohort 2, n=278, and the Cancer Genome Atlas cohort, n=375) were analyzed for YTHDF1 expression. Function of YTHDF1 in GC was determined in GC cell lines. Role of YTHDF1 in antitumor immunity was investigated in allograft models.
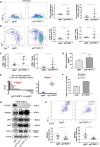
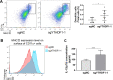
Results: YTHDF1 is upregulated in GC compared with adjacent normal tissues, and high YTHDF1 expression was correlated with poor survival of patients with GC at mRNA (p=0.016) and protein levels (p=0.039). Loss of YTHDF1 in human (AGS, BGC823, MKN74) or mouse (YTN16) GC cell lines inhibited cell growth and colony formation in vitro. Strikingly, syngeneic YTN16 tumors with loss of YTHDF1 underwent complete remission in immunocompetent mice, while a lesser effect was found in immunodeficient mice. Consistently, YTHDF1 loss in GC tumors led to recruitment of mature dendritic cells (DCs) with increased MHCII expression and interleukin-12 (IL-12) secretion, which in turn, promoted CD4+ and CD8+ T cells infiltration with increased interferon-γ (IFN-γ) secretion. Loss of YTHDF1 mediated the overexpression of IFN-γ receptor 1 and JAK/STAT1 signaling pathway in tumor cells, which might contribute to restored sensitivity to antitumor immunity. In addition, pre-emptive exposure of YTN16 tumors with YTHDF1 loss triggered a potent antitumor immune response on rechallenge with wild-type YTN16 cells, implying that YTHDF1 loss induced a lasting systemic antitumor immunity.
Conclusions: YTHDF1 is overexpressed in GC and promotes GC by inducing cell proliferation and repression of DCs-mediated antitumor immune response. YTHDF1 is a promising therapeutic target for GC treatment.
Keywords: dendritic cells; immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
