Agonist-Specific Regulation of G Protein-Coupled Receptors after Chronic Opioid Treatment
- PMID: 35193934
- PMCID: PMC9092468
- DOI: 10.1124/molpharm.121.000453
Agonist-Specific Regulation of G Protein-Coupled Receptors after Chronic Opioid Treatment
Abstract
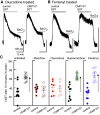
Chronic treatment of animals with morphine results in a long lasting cellular tolerance in the locus coeruleus and alters the kinase dependent desensitization of opioid and nonopioid G protein-coupled receptors (GPCRs). This study examined the development of tolerance and altered regulation of kinase activity after chronic treatment of animals with clinically relevant opioids that differ in efficacy at the µ-opioid receptors (MOR). In slices from oxycodone treated animals, no tolerance to opioids was observed when measuring the MOR induced increase in potassium conductance, but the G protein receptor kinase 2/3 blocker, compound 101, no longer inhibited desensitization of somatostatin (SST) receptors. Chronic fentanyl treatment induced a rightward shift in the concentration response to [Met5]enkephalin, but there was no change in the kinase regulation of desensitization of the SST receptor. When total phosphorylation deficient MORs that block desensitization, internalization, and tolerance were virally expressed, chronic treatment with fentanyl resulted in the altered kinase regulation of SST receptors. The results suggest that sustained opioid receptor signaling initiates the process that results in altered kinase regulation of not only opioid receptors, but also other GPCRs. This study highlights two very distinct downstream adaptive processes that are specifically regulated by an agonist dependent mechanism. SIGNIFICANCE STATEMENT: Persistent signaling of MORs results in altered kinase regulation of nonopioid GPCRs after chronic treatment with morphine and oxycodone. Profound tolerance develops after chronic treatment with fentanyl without affecting kinase regulation. The homeostatic change in the kinase regulation of nonopioid GPCRs could account for the systems level in vivo development of tolerance that is seen with opioid agonists, such as morphine and oxycodone, that develop more rapidly than the tolerance induced by efficacious agonists, such as fentanyl and etorphine.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

