A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia
- PMID: 35194166
- PMCID: PMC9126816
- DOI: 10.1038/s41380-022-01456-3
A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia
Abstract
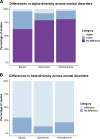
The emerging understanding of gut microbiota as 'metabolic machinery' influencing many aspects of physiology has gained substantial attention in the field of psychiatry. This is largely due to the many overlapping pathophysiological mechanisms associated with both the potential functionality of the gut microbiota and the biological mechanisms thought to be underpinning mental disorders. In this systematic review, we synthesised the current literature investigating differences in gut microbiota composition in people with the major psychiatric disorders, major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia (SZ), compared to 'healthy' controls. We also explored gut microbiota composition across disorders in an attempt to elucidate potential commonalities in the microbial signatures associated with these mental disorders. Following the PRISMA guidelines, databases were searched from inception through to December 2021. We identified 44 studies (including a total of 2510 psychiatric cases and 2407 controls) that met inclusion criteria, of which 24 investigated gut microbiota composition in MDD, seven investigated gut microbiota composition in BD, and 15 investigated gut microbiota composition in SZ. Our syntheses provide no strong evidence for a difference in the number or distribution (α-diversity) of bacteria in those with a mental disorder compared to controls. However, studies were relatively consistent in reporting differences in overall community composition (β-diversity) in people with and without mental disorders. Our syntheses also identified specific bacterial taxa commonly associated with mental disorders, including lower levels of bacterial genera that produce short-chain fatty acids (e.g. butyrate), higher levels of lactic acid-producing bacteria, and higher levels of bacteria associated with glutamate and GABA metabolism. We also observed substantial heterogeneity across studies with regards to methodologies and reporting. Further prospective and experimental research using new tools and robust guidelines hold promise for improving our understanding of the role of the gut microbiota in mental and brain health and the development of interventions based on modification of gut microbiota.
© 2022. The Author(s).
Conflict of interest statement
MOH has a financial interest in Prevatex Pty Ltd, a company developing probiotic-based biotherapeutics. AL is a named inventor on a patent relating to
Figures
References
-
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80–101. - PubMed
-
- Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

