Cumulative Clinical Benefits of Biologics in the Treatment of Patients with Moderate-to-Severe Psoriasis over 1 Year: a Network Meta-Analysis
- PMID: 35195887
- PMCID: PMC8941028
- DOI: 10.1007/s13555-022-00690-5
Cumulative Clinical Benefits of Biologics in the Treatment of Patients with Moderate-to-Severe Psoriasis over 1 Year: a Network Meta-Analysis
Abstract
Introduction: Both early clinical improvement and long-term maintenance of clinical efficacy of treatments matter to patients with psoriasis. We compared cumulative clinical benefits of treatment with biologics over 1 year based on the area under the curve (AUC) for Psoriasis Area and Severity Index (PASI) 100 and PASI 90 responses in patients with moderate-to-severe psoriasis using a network meta-analysis (NMA).
Methods: Published phase 3 randomized, placebo- or active-controlled clinical trial data for biologics approved for the treatment of moderate-to-severe psoriasis were obtained from a systematic literature review up to 30 September 2020. Eighteen clinical trials that included data from baseline to 48 or 52 weeks where AUC could be calculated were included. Data were compared using a fixed-effect model with a separate random-effect baseline model to account for effects of the placebo arm. Cumulative clinical benefit was estimated using the AUC for PASI 100 and PASI 90 responses (complete and almost-complete skin clearance, respectively). Normalized AUC was compared using Bayesian NMA. Cumulative days of response were calculated using normalized AUC and study duration.
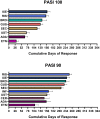
Results: Interleukin (IL)-17 and IL-23 inhibitors demonstrated greater cumulative clinical benefits for both PASI 100 and PASI 90 versus IL-12/23 and tumor necrosis factor inhibitors. Over 52 weeks, cumulative days with PASI 100 were greatest with ixekizumab [158.7 (95% credible interval, 147.4, 170.0) days] followed by risankizumab [154.0 (144.9, 163.4) days]; PASI 90 days were greatest with risankizumab [249.3 (239.5, 259.2) days] followed by ixekizumab [238.8 (227.1, 250.8) days]. Both ixekizumab and risankizumab showed greater cumulative days with PASI 100 or PASI 90 responses versus secukinumab [117.9 (110.7, 125.2) and 215.5 (208.2, 223.1) days, respectively] and greater cumulative days with PASI 100 versus guselkumab [130.7 (120.5, 140.9) days].
Conclusion: For complete and almost-complete skin clearance, ixekizumab and risankizumab provided the greatest cumulative clinical benefits over 1 year.
Keywords: Area under the curve; Biologics; Cumulative benefit; Ixekizumab; NMA; Psoriasis.
© 2022. The Author(s).
Figures

References
-
- Gorelick J, Shrom D, Sikand K, Renda L, Burge R, Dworkin C, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross-sectional patient survey. Dermatol Ther (Heidelb) 2019;9(4):785–797. doi: 10.1007/s13555-019-00334-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

