Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia
- PMID: 35196021
- PMCID: PMC9210521
- DOI: 10.1126/scitranslmed.abm1375
Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia
Abstract
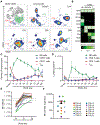
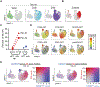
Natural killer (NK) cells are innate lymphoid cells that eliminate cancer cells, produce cytokines, and are being investigated as a nascent cellular immunotherapy. Impaired NK cell function, expansion, and persistence remain key challenges for optimal clinical translation. One promising strategy to overcome these challenges is cytokine-induced memory-like (ML) differentiation, whereby NK cells acquire enhanced antitumor function after stimulation with interleukin-12 (IL-12), IL-15, and IL-18. Here, reduced-intensity conditioning (RIC) for HLA-haploidentical hematopoietic cell transplantation (HCT) was augmented with same-donor ML NK cells on day +7 and 3 weeks of N-803 (IL-15 superagonist) to treat patients with relapsed/refractory acute myeloid leukemia (AML) in a clinical trial (NCT02782546). In 15 patients, donor ML NK cells were well tolerated, and 87% of patients achieved a composite complete response at day +28, which corresponded with clearing high-risk mutations, including TP53 variants. NK cells were the major blood lymphocytes for 2 months after HCT with 1104-fold expansion (over 1 to 2 weeks). Phenotypic and transcriptional analyses identified donor ML NK cells as distinct from conventional NK cells and showed that ML NK cells persisted for over 2 months. ML NK cells expressed CD16, CD57, and high granzyme B and perforin, along with a unique transcription factor profile. ML NK cells differentiated in patients had enhanced ex vivo function compared to conventional NK cells from both patients and healthy donors. Overall, same-donor ML NK cell therapy with 3 weeks of N-803 support safely augmented RIC haplo-HCT for AML.
Figures





References
-
- Estey EH, Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol 93, 1267–1291 (2018). - PubMed
-
- Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, Mielcarek M, Estey EH, Appelbaum FR, Walter RB, Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J. Clin. Oncol 34, 329–336 (2016). - PMC - PubMed
-
- Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, Maziarz RT, Warlick ED, Fernandez HF, Alyea EP, Hamadani M, Bashey A, Giralt S, Geller NL, Leifer E, Le-Rademacher J, Mendizabal AM, Horowitz MM, Deeg HJ, Horwitz ME, Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J. Clin. Oncol 35, 1154–1161 (2017). - PMC - PubMed
-
- Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, Ray K, O’Donnell PV, Chakrabarti S, Donnell PVO, Chakrabarti S, O’Donnell PV, Chakrabarti S, Improved outcome of refractory/relapsed acute myeloid leukemia after post-transplantation cyclophosphamide-based haploidentical transplantation with myeloablative conditioning and early prophylactic granulocyte colony-stimulating factor–mobilized donor lymphocyte infusions. Biol. Blood Marrow Transplant 22, 1867–1873 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

