Activation of PDGFRA signaling contributes to filamin C-related arrhythmogenic cardiomyopathy
- PMID: 35196083
- PMCID: PMC8865769
- DOI: 10.1126/sciadv.abk0052
Activation of PDGFRA signaling contributes to filamin C-related arrhythmogenic cardiomyopathy
Abstract
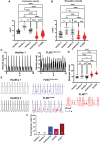
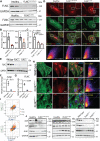
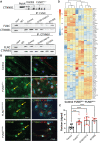
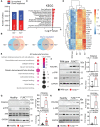
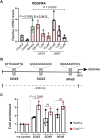
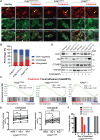
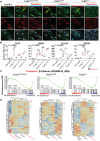
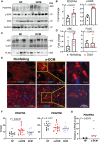
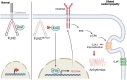
FLNC truncating mutations (FLNCtv) are prevalent causes of inherited dilated cardiomyopathy (DCM), with a high risk of developing arrhythmogenic cardiomyopathy. We investigated the molecular mechanisms of mutant FLNC in the pathogenesis of arrhythmogenic DCM (a-DCM) using patient-specific induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). We demonstrated that iPSC-CMs from two patients with different FLNCtv mutations displayed arrhythmias and impaired contraction. FLNC ablation induced a similar phenotype, suggesting that FLNCtv are loss-of-function mutations. Coimmunoprecipitation and proteomic analysis identified β-catenin (CTNNB1) as a downstream target. FLNC deficiency induced nuclear translocation of CTNNB1 and subsequently activated the platelet-derived growth factor receptor alpha (PDGFRA) pathway, which were also observed in human hearts with a-DCM and FLNCtv. Treatment with the PDGFRA inhibitor, crenolanib, improved contractile function of patient iPSC-CMs. Collectively, our findings suggest that PDGFRA signaling is implicated in the pathogenesis, and inhibition of this pathway is a potential therapeutic strategy in FLNC-related cardiomyopathies.
Figures
References
-
- Towbin J. A., Lorts A., Arrhythmias and dilated cardiomyopathy. J. Am. Coll. Cardiol. 57, 2169–2171 (2011). - PubMed
-
- McKenna W. J., Maron B. J., Thiene G., Classification, epidemiology, and global burden of cardiomyopathies. Circ. Res. 121, 722–730 (2017). - PubMed
-
- Hershberger R. E., Hedges D. J., Morales A., Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 10, 531–547 (2013). - PubMed
Grants and funding
- R01 HL113006/HL/NHLBI NIH HHS/United States
- R01 HL109209/HL/NHLBI NIH HHS/United States
- R01 HL141851/HL/NHLBI NIH HHS/United States
- R01 HL123968/HL/NHLBI NIH HHS/United States
- R01 HL069071/HL/NHLBI NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- R01 HL126527/HL/NHLBI NIH HHS/United States
- K23 HL067915/HL/NHLBI NIH HHS/United States
- R01 HL147064/HL/NHLBI NIH HHS/United States
- R01 HL130020/HL/NHLBI NIH HHS/United States
- P01 HL141084/HL/NHLBI NIH HHS/United States
- R01 HL116906/HL/NHLBI NIH HHS/United States
- 17GRNT33670495/AHA/American Heart Association-American Stroke Association/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

