Vaccine-Induced, High-Magnitude HIV Env-Specific Antibodies with Fc-Mediated Effector Functions Are Insufficient to Protect Infant Rhesus Macaques against Oral SHIV Infection
- PMID: 35196125
- PMCID: PMC8865927
- DOI: 10.1128/msphere.00839-21
Vaccine-Induced, High-Magnitude HIV Env-Specific Antibodies with Fc-Mediated Effector Functions Are Insufficient to Protect Infant Rhesus Macaques against Oral SHIV Infection
Abstract
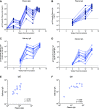
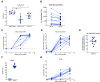
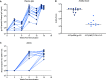
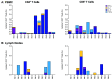
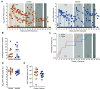
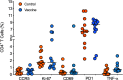
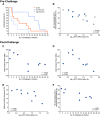
Improved access to antiretroviral therapy (ART) and antenatal care has significantly reduced in utero and peripartum mother-to-child human immunodeficiency virus (HIV) transmission. However, as breast milk transmission of HIV still occurs at an unacceptable rate, there remains a need to develop an effective vaccine for the pediatric population. Previously, we compared different HIV vaccine strategies, intervals, and adjuvants in infant rhesus macaques to optimize the induction of HIV envelope (Env)-specific antibodies with Fc-mediated effector function. In this study, we tested the efficacy of an optimized vaccine regimen against oral simian-human immunodeficiency virus (SHIV) acquisition in infant macaques. Twelve animals were immunized with 1086.c gp120 protein adjuvanted with 3M-052 in stable emulsion and modified vaccinia Ankara (MVA) virus expressing 1086.c HIV Env. Twelve control animals were immunized with empty MVA. The vaccine prime was given within 10 days of birth, with booster doses being administered at weeks 6 and 12. The vaccine regimen induced Env-specific plasma IgG antibodies capable of antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP). Beginning at week 15, infants were exposed orally to escalating doses of heterologous SHIV-1157(QNE)Y173H once a week until infected. Despite the induction of strong Fc-mediated antibody responses, the vaccine regimen did not reduce the risk of infection or time to acquisition compared to controls. However, among vaccinated animals, ADCC postvaccination and postinfection was associated with reduced peak viremia. Thus, nonneutralizing Env-specific antibodies with Fc effector function elicited by this vaccine regimen were insufficient for protection against heterologous oral SHIV infection shortly after the final immunization but may have contributed to control of viremia. IMPORTANCE Women of childbearing age are three times more likely to contract HIV infection than their male counterparts. Poor HIV testing rates coupled with low adherence to antiretroviral therapy (ART) result in a high risk of mother-to-infant HIV transmission, especially during the breastfeeding period. A preventative vaccine could curb pediatric HIV infections, reduce potential health sequalae, and prevent the need for lifelong ART in this population. The results of the current study imply that the HIV Env-specific IgG antibodies elicited by this candidate vaccine regimen, despite a high magnitude of Fc-mediated effector function but a lack of neutralizing antibodies and polyfunctional T cell responses, were insufficient to protect infant rhesus macaques against oral virus acquisition.
Keywords: ADCC; Fc-mediated antibody function; pediatric HIV vaccine; rhesus macaque.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- UNAIDS. 2018. Global HIV & AIDS statistics—2018 fact sheet. https://www.unaids.org.
-
- Haas AD, Msukwa MT, Egger M, Tenthani L, Tweya H, Jahn A, Gadabu OJ, Tal K, Salazar-Vizcaya L, Estill J, Spoerri A, Phiri N, Chimbwandira F, van Oosterhout JJ, Keiser O. 2016. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi’s Option B+ program. Clin Infect Dis 63:1227–1235. doi:10.1093/cid/ciw500. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

