Treatments targeting autophagy ameliorate the age-related macular degeneration phenotype in mice lacking APOE (apolipoprotein E)
- PMID: 35196199
- PMCID: PMC9542759
- DOI: 10.1080/15548627.2022.2034131
Treatments targeting autophagy ameliorate the age-related macular degeneration phenotype in mice lacking APOE (apolipoprotein E)
Abstract
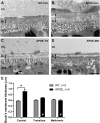
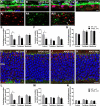
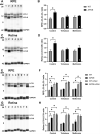
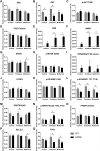
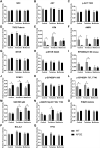
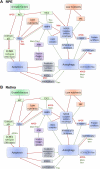
Age-related macular degeneration (AMD) is a leading cause of vision loss with recent evidence indicating an important role for macroautophagy/autophagy in disease progression. In this study we investigate the efficacy of targeting autophagy for slowing dysfunction in a mouse model with features of early AMD. Mice lacking APOE (apolipoprotein E; B6.129P2-Apoetm1UncJ/Arc) and C57BL/6 J- (wild-type, WT) mice were treated with metformin or trehalose in the drinking water from 5 months of age and the ocular phenotype investigated at 13 months. Control mice received normal drinking water. APOE-control mice had reduced retinal function and thickening of Bruch's membrane consistent with an early AMD phenotype. Immunohistochemical labeling showed reductions in MAP1LC3B/LC3 (microtubule-associated protein 1 light chain 3 beta) and LAMP1 (lysosomal-associated membrane protein 1) labeling in the photoreceptors and retinal pigment epithelium (RPE). This correlated with increased LC3-II:LC3-I ratio and alterations in protein expression in multiple autophagy pathways measured by reverse phase protein array, suggesting autophagy was slowed. Treatment of APOE-mice with metformin or trehalose ameliorated the loss of retinal function and reduced Bruch's membrane thickening, enhancing LC3 and LAMP1 labeling in the ocular tissues and restoring LC3-II:LC3-I ratio to WT levels. Protein analysis indicated that both treatments boost ATM-AMPK driven autophagy. Additionally, trehalose increased p-MAPK14/p38 to enhance autophagy. Our study shows that treatments targeting pathways to enhance autophagy have the potential for treating early AMD and provide support for the use of metformin, which has been found to reduce the risk of AMD development in human patients.Abbreviations:AMD: age-related macular degeneration; AMPK: 5' adenosine monophosphate-activated protein kinase APOE: apolipoprotein E; ATM: ataxia telangiectasia mutated; BCL2L1/Bcl-xL: BCL2-like 1; DAPI: 4'-6-diamidino-2-phenylindole; ERG: electroretinogram; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GCL: ganglion cell layer; INL: inner nuclear layer; IPL: inner plexiform layer; IS/OS: inner and outer photoreceptor segments; LAMP1: lysosomal-associated membrane protein 1; MAP1LC3B/LC3: microtubule-associated protein 1 light chain 3 beta; MTOR: mechanistic target of rapamycin kinase; OCT: optical coherence tomography; ONL: outer nuclear layer; OPs: oscillatory potentials; p-EIF4EBP1: phosphorylated eukaryotic translation initiation factor 4E binding protein 1; p-MAPK14/p38: phosphorylated mitogen-activated protein kinase 14; RPE: retinal pigment epithelium; RPS6KB/p70 S6 kinase: ribosomal protein S6 kinase; SQSTM1/p62: sequestosome 1; TP53/TRP53/p53: tumor related protein 53; TSC2: TSC complex subunit 2; WT: wild type.
Keywords: B6.129P2-Apoetm1UncJ; bruch’s membrane; metformin; retina; retinal pigment epithelium; trehalose.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738. - PubMed
-
- Adler R, Curcio C, Hicks D, et al. Cell death in age-related macular degeneration. Mol Vis. 1999;5:31. - PubMed
-
- Owens SL, Guymer RH, Gross-Jendroska M, et al. Fluorescein angiographic abnormalities after prophylactic macular photocoagulation for high-risk age-related maculopathy. Am J Ophthalmol. 1999;127(6):681–687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
