Seizures initiate in zones of relative hyperexcitation in a zebrafish epilepsy model
- PMID: 35196385
- PMCID: PMC9612797
- DOI: 10.1093/brain/awac073
Seizures initiate in zones of relative hyperexcitation in a zebrafish epilepsy model
Abstract
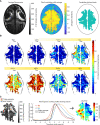
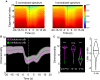
Seizures are thought to arise from an imbalance of excitatory and inhibitory neuronal activity. While most classical studies suggest excessive excitatory neural activity plays a generative role, some recent findings challenge this view and instead argue that excessive activity in inhibitory neurons initiates seizures. We investigated this question of imbalance in a zebrafish seizure model with two-photon imaging of excitatory and inhibitory neuronal activity throughout the brain using a nuclear-localized calcium sensor. We found that seizures consistently initiated in circumscribed zones of the midbrain before propagating to other brain regions. Excitatory neurons were both more prevalent and more likely to be recruited than inhibitory neurons in initiation as compared with propagation zones. These findings support a mechanistic picture whereby seizures initiate in a region of hyperexcitation, then propagate more broadly once inhibitory restraint in the surround is overcome.
Keywords: E/I balance; calcium imaging; ictogenesis; seizure initiation; seizure propagation.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes—New knowledge of cortical feedback-systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. - PubMed
-
- Dichter MA, Ayala GF. Cellular mechanisms of epilepsy—A status-report. Science. 1987;237(4811):157–164. - PubMed
-
- Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy—Ictal manifestations. Exp Neurol. 1964;9(4):305–326. - PubMed
-
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

