Study of humoral and cellular immunity in vaccinated with mRNA-1273
- PMID: 35196403
- PMCID: PMC9111507
- DOI: 10.1111/apm.13215
Study of humoral and cellular immunity in vaccinated with mRNA-1273
Abstract
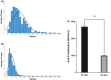
The new vaccines against SARS-CoV-2 have raised a lot of expectations about their ability to induce immunity and the duration of this. This is the case of mRNA vaccines such as Moderna's mRNA-1273. Therefore, it is necessary to study the humoral and cellular immunity generated by these vaccines. Our objectives are determining what is the normal response of antibody production, and what is the level of protective antibodies and monitoring patients in case of subsequent infection with COVID-19. We present the first results of a longitudinal study of the humoral response in 601 health workers vaccinated with Moderna. The results show a humoral immunity at 90 days after the second dose of 100%, with a strong decrease between the levels of circulating anti-S IgG antibodies between days 30 and 90 post-vaccination. Observing a steeper decline in those who had higher titles at the beginning. In addition, we present a cellular response of 86% at three months after the second dose, which is related to low humoral response.
Keywords: Moderna vaccine; SARS-CoV-2; humoral response; mRNA vaccines; mRNA-1273 vaccine.
© 2022 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Medical Microbiology and Pathology.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization . Coronavirus disease. Coronavirus Dis Situat Rep – 119 2020;2019:2633. 10.1001/jama.2020.2633 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

