Mechanisms of Resistance to Noncovalent Bruton's Tyrosine Kinase Inhibitors
- PMID: 35196427
- PMCID: PMC9074143
- DOI: 10.1056/NEJMoa2114110
Mechanisms of Resistance to Noncovalent Bruton's Tyrosine Kinase Inhibitors
Abstract
Background: Covalent (irreversible) Bruton's tyrosine kinase (BTK) inhibitors have transformed the treatment of multiple B-cell cancers, especially chronic lymphocytic leukemia (CLL). However, resistance can arise through multiple mechanisms, including acquired mutations in BTK at residue C481, the binding site of covalent BTK inhibitors. Noncovalent (reversible) BTK inhibitors overcome this mechanism and other sources of resistance, but the mechanisms of resistance to these therapies are currently not well understood.
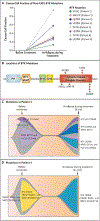
Methods: We performed genomic analyses of pretreatment specimens as well as specimens obtained at the time of disease progression from patients with CLL who had been treated with the noncovalent BTK inhibitor pirtobrutinib. Structural modeling, BTK-binding assays, and cell-based assays were conducted to study mutations that confer resistance to noncovalent BTK inhibitors.
Results: Among 55 treated patients, we identified 9 patients with relapsed or refractory CLL and acquired mechanisms of genetic resistance to pirtobrutinib. We found mutations (V416L, A428D, M437R, T474I, and L528W) that were clustered in the kinase domain of BTK and that conferred resistance to both noncovalent BTK inhibitors and certain covalent BTK inhibitors. Mutations in BTK or phospholipase C gamma 2 (PLCγ2), a signaling molecule and downstream substrate of BTK, were found in all 9 patients. Transcriptional activation reflecting B-cell-receptor signaling persisted despite continued therapy with noncovalent BTK inhibitors.
Conclusions: Resistance to noncovalent BTK inhibitors arose through on-target BTK mutations and downstream PLCγ2 mutations that allowed escape from BTK inhibition. A proportion of these mutations also conferred resistance across clinically approved covalent BTK inhibitors. These data suggested new mechanisms of genomic escape from established covalent and novel noncovalent BTK inhibitors. (Funded by the American Society of Hematology and others.).
Copyright © 2022 Massachusetts Medical Society.
Figures


Comment in
-
Acquired BTK mutations associated with resistance to noncovalent BTK inhibitors.Blood Adv. 2023 Oct 10;7(19):5698-5702. doi: 10.1182/bloodadvances.2022008955. Blood Adv. 2023. PMID: 36661329 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA229086/CA/NCI NIH HHS/United States
- T32 CA009207/CA/NCI NIH HHS/United States
- R01 CA228135/CA/NCI NIH HHS/United States
- P50 CA254838/CA/NCI NIH HHS/United States
- F31 CA275378/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 HL159175/HL/NHLBI NIH HHS/United States
- R01 CA242020/CA/NCI NIH HHS/United States
- R01 CA173636/CA/NCI NIH HHS/United States
- K08 CA230319/CA/NCI NIH HHS/United States
- R01 CA216421/CA/NCI NIH HHS/United States
- R01CA216421, R01CA173636, R01CA228135, P01CA229086/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
