Tanshinones: Leading the way into Lamiaceae labdane-related diterpenoid biosynthesis
- PMID: 35196638
- PMCID: PMC8940693
- DOI: 10.1016/j.pbi.2022.102189
Tanshinones: Leading the way into Lamiaceae labdane-related diterpenoid biosynthesis
Abstract
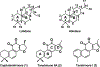
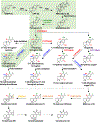
Tanshinones are the bioactive diterpenoid constituents of the traditional Chinese medicinal herb Danshen (Salvia miltiorrhiza), and are examples of the phenolic abietanes widely found within the Lamiaceae plant family. Due to the significant interest in these labdane-related diterpenoid natural products, their biosynthesis has been intensively investigated. In addition to providing the basis for metabolic engineering efforts, this work further yielded pioneering insights into labdane-related diterpenoid biosynthesis in the Lamiaceae more broadly. This includes stereochemical foreshadowing of aromatization, with novel protein domain loss in the relevant diterpene synthase, as well as broader phylogenetic conservation of the relevant enzymes. Beyond such summary of more widespread metabolism, formation of the furan ring that characterizes the tanshinones also has been recently elucidated. Nevertheless, the biocatalysts for the pair of demethylations remain unknown, and the intriguing potential connection of these reactions to the further aromatization observed in the tanshinones are speculated upon here.
Keywords: 2-oxoglutarate dependent dioxygenases; Cytochromes P450; Short-chain alcohol dehydrogenases/reductases; Terpene synthases.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Expansion within the CYP71D subfamily drives the heterocyclization of tanshinones synthesis in Salvia miltiorrhiza.Nat Commun. 2021 Jan 29;12(1):685. doi: 10.1038/s41467-021-20959-1. Nat Commun. 2021. PMID: 33514704 Free PMC article.
-
Targeted mutagenesis of CYP76AK2 and CYP76AK3 in Salvia miltiorrhiza reveals their roles in tanshinones biosynthetic pathway.Int J Biol Macromol. 2021 Oct 31;189:455-463. doi: 10.1016/j.ijbiomac.2021.08.112. Epub 2021 Aug 19. Int J Biol Macromol. 2021. PMID: 34419551
-
CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12108-13. doi: 10.1073/pnas.1218061110. Epub 2013 Jun 28. Proc Natl Acad Sci U S A. 2013. PMID: 23812755 Free PMC article.
-
Biosynthesis and regulatory mechanism of tanshinones and phenolic acids in Salvia miltiorrhiza.Plant J. 2025 Jul;123(2):e70358. doi: 10.1111/tpj.70358. Plant J. 2025. PMID: 40720694 Review.
-
Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures.Appl Microbiol Biotechnol. 2010 Sep;88(2):437-49. doi: 10.1007/s00253-010-2797-7. Epub 2010 Aug 7. Appl Microbiol Biotechnol. 2010. PMID: 20694462 Review.
Cited by
-
Functional Characterization of a 2OGD Involved in Abietane-Type Diterpenoids Biosynthetic Pathway in Salvia miltiorrhiza.Front Plant Sci. 2022 Jul 7;13:947674. doi: 10.3389/fpls.2022.947674. eCollection 2022. Front Plant Sci. 2022. PMID: 35873989 Free PMC article.
-
Biosynthesis-based metabolomics analysis reveals chemical diversity between two Salvia species.Front Plant Sci. 2025 Jul 4;16:1613313. doi: 10.3389/fpls.2025.1613313. eCollection 2025. Front Plant Sci. 2025. PMID: 40688688 Free PMC article.
-
From functional plasticity of two diterpene synthases (IrTPS2/IrKSL3a) to enzyme evolution.ACS Catal. 2024 Mar 1;14(5):2959-2970. doi: 10.1021/acscatal.3c05918. Epub 2024 Feb 12. ACS Catal. 2024. PMID: 40746662 Free PMC article.
-
Dynamic evolution of terpenoid biosynthesis in the Lamiaceae.Mol Plant. 2023 Jun 5;16(6):963-965. doi: 10.1016/j.molp.2023.04.012. Epub 2023 Apr 27. Mol Plant. 2023. PMID: 37118894 Free PMC article. No abstract available.
-
Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants.Biomolecules. 2023 Nov 30;13(12):1725. doi: 10.3390/biom13121725. Biomolecules. 2023. PMID: 38136596 Free PMC article. Review.
References
-
- Mei X, Cao Y, Che Y, Li J, Shang Z, Zhao W, Qiao Y, Zhang J: Danshen: a phytochemical and pharmacological overview. Chin J Nat Med 2019, 17:59–80. - PubMed
-
- Chinese Pharmacopoeia Commission: Pharmacopoeia of the People’s Republic of China. Vol. 1. 2020.
-
- Gonzalez MA: Aromatic abietane diterpenoids: their biological activity and synthesis. Nat Prod Rep 2015, 32:684–704. - PubMed
-
- Wang X, Morris-Natschke SL, Lee KH: New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev 2007, 27:133–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

