SARS-CoV-2 vaccination elicits unconventional IgM specific responses in naïve and previously COVID-19-infected individuals
- PMID: 35196644
- PMCID: PMC8858081
- DOI: 10.1016/j.ebiom.2022.103888
SARS-CoV-2 vaccination elicits unconventional IgM specific responses in naïve and previously COVID-19-infected individuals
Abstract
Background: Currently, evaluation of the IgG antibodies specific for the SARS-CoV-2 Spike protein following vaccination is used worldwide to estimate vaccine response. Limited data are available on vaccine-elicited IgM antibodies and their potential implication in immunity to SARS-CoV-2.
Methods: We performed a longitudinal study to quantify anti-S SARS-CoV-2 IgG and IgM (IgG-S and IgM-S) in health care worker (HCW) recipients of the BNT162b2 vaccine. Samples were collected before administration (T0), at the second dose (T1) and three weeks after T1 (T2). The cohort included 1584 immunologically naïve to SARS-CoV-2 (IN) and 289 with history of previous infection (PI).
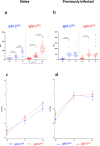
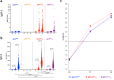
Findings: IN showed three patterns of responses: (a) IgG positive/IgM negative (36.1%), (b) coordinated IgM-S/IgG-S responses appearing at T1 (37.4%) and (c) IgM appearing after IgG (26.3%). Coordinated IgM-S/IgG-S responses were associated with higher IgG titres. In IgM-S positive PI, 64.5% were IgM-S positive before vaccination, whereas 32% and 3.5% developed IgM-S after the first and second vaccine dose, respectively. IgM-S positive sera had higher pseudovirus neutralization titres compared to the IgM-S negative.
Interpretation: Coordinated expression of IgG-S and IgM-S after vaccination was associated with a significantly more efficient response in both antibody levels and virus-neutralizing activity. The unconventional IgG-S positive/IgM-S negative responses may suggest a recruitment of cross coronaviruses immunity by vaccination, warranting further investigation.
Funding: Italian Ministry of Health under "Fondi Ricerca Corrente"- L1P5 and "Progetto Ricerca Finalizzata COVID-2020-12371675"; FUR 2020 Department of Excellence 2018-2022, MIUR, Italy; The Brain Research Foundation Verona.
Keywords: BTN162b2 vaccine; COVID-19; IgG; IgM; SARS-CoV-2; Spike.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
IgM responses following SARS-CoV-2 vaccination: insights into protective and pre-existing immunity.EBioMedicine. 2022 Mar;77:103922. doi: 10.1016/j.ebiom.2022.103922. Epub 2022 Mar 5. EBioMedicine. 2022. PMID: 35259573 Free PMC article. No abstract available.
References
-
- Long Q.X., Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

