Spread of Gamma (P.1) Sub-Lineages Carrying Spike Mutations Close to the Furin Cleavage Site and Deletions in the N-Terminal Domain Drives Ongoing Transmission of SARS-CoV-2 in Amazonas, Brazil
- PMID: 35196783
- PMCID: PMC8865440
- DOI: 10.1128/spectrum.02366-21
Spread of Gamma (P.1) Sub-Lineages Carrying Spike Mutations Close to the Furin Cleavage Site and Deletions in the N-Terminal Domain Drives Ongoing Transmission of SARS-CoV-2 in Amazonas, Brazil
Abstract
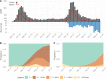
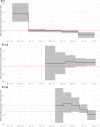
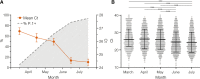
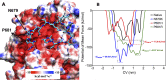
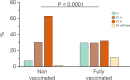
The Amazonas was one of the most heavily affected Brazilian states by the COVID-19 epidemic. Despite a large number of infected people, particularly during the second wave associated with the spread of the Variant of Concern (VOC) Gamma (lineage P.1), SARS-CoV-2 continues to circulate in the Amazonas. To understand how SARS-CoV-2 persisted in a human population with a high immunity barrier, we generated 1,188 SARS-CoV-2 whole-genome sequences from individuals diagnosed in the Amazonas state from 1st January to 6th July 2021, of which 38 were vaccine breakthrough infections. Our study reveals a sharp increase in the relative prevalence of Gamma plus (P.1+) variants, designated Pango Lineages P.1.3 to P.1.6, harboring two types of additional Spike changes: deletions in the N-terminal (NTD) domain (particularly Δ144 or Δ141-144) associated with resistance to anti-NTD neutralizing antibodies or mutations at the S1/S2 junction (N679K or P681H) that probably enhance the binding affinity to the furin cleavage site, as suggested by our molecular dynamics simulations. As lineages P.1.4 (S:N679K) and P.1.6 (S:P681H) expanded (Re > 1) from March to July 2021, the lineage P.1 declined (Re < 1) and the median Ct value of SARS-CoV-2 positive cases in Amazonas significantly decreases. Still, we did not find an increased incidence of P.1+ variants among breakthrough cases of fully vaccinated patients (71%) in comparison to unvaccinated individuals (93%). This evidence supports that the ongoing endemic transmission of SARS-CoV-2 in the Amazonas is driven by the spread of new local Gamma/P.1 sublineages that are more transmissible, although not more efficient to evade vaccine-elicited immunity than the parental VOC. Finally, as SARS-CoV-2 continues to spread in human populations with a declining density of susceptible hosts, the risk of selecting more infectious variants or antibody evasion mutations is expected to increase. IMPORTANCE The continuous evolution of SARS-CoV-2 is an expected phenomenon that will continue to happen due to the high number of cases worldwide. The present study analyzed how a Variant of Concern (VOC) could still circulate in a population hardly affected by two COVID-19 waves and with vaccination in progress. Our results showed that the answer behind that was a new generation of Gamma-like viruses, which emerged locally carrying mutations that made it more transmissible and more capable of spreading, partially evading prior immunity triggered by natural infections or vaccines. With thousands of new cases daily, the current pandemics scenario suggests that SARS-CoV-2 will continue to evolve and efforts to reduce the number of infected subjects, including global equitable access to COVID-19 vaccines, are mandatory. Thus, until the end of pandemics, the SARS-CoV-2 genomic surveillance will be an essential tool to better understand the drivers of the viral evolutionary process.
Keywords: Brazil; COVID-19; SARS-CoV-2; coronavirus; variant gamma; virus evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fundação de Vigilância em Saúde do Amazonas. 2021. Boletim diaria COVID-19 no Amazonas 15–06-2021. https://www.fvs.am.gov.br/noticias_view/6264. Accessed July 03, 2021.
-
- Naveca FG, Nascimento V, Souza VCd, Corado AL, Nascimento F, Silva G, Costa Á, Duarte D, Pessoa K, Mejía M, Brandão MJ, Jesus M, Gonçalves L, Costa CFd, Sampaio V, Barros D, Silva M, Mattos T, Pontes G, Abdalla L, Santos JH, Arantes I, Dezordi FZ, Siqueira MM, Wallau GL, Resende PC, Delatorre E, Gräf T, Bello G. 2021. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med 27:1230–1238. doi: 10.1038/s41591-021-01378-7. - DOI - PubMed
-
- Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, Crispim MAE, Sales FCS, Hawryluk I, McCrone JT, Hulswit RJG, Franco LAM, Ramundo MS, de Jesus JG, Andrade PS, Coletti TM, Ferreira GM, Silva CAM, Manuli ER, Pereira RHM, Peixoto PS, Kraemer MUG, Gaburo N, Jr, Camilo CDC, Hoeltgebaum H, Souza WM, Rocha EC, de Souza LM, de Pinho MC, Araujo LJT, Malta FSV, de Lima AB, Silva JDP, Zauli DAG, Ferreira ACS, Schnekenberg RP, Laydon DJ, Walker PGT, Schluter HM, Dos Santos ALP, Vidal MS, Del Caro VS, Filho RMF, Dos Santos HM, Aguiar RS, Proenca-Modena JL, Nelson B, Hay JA, Monod M, Miscouridou X, et al. 2021. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 372:815–821. doi: 10.1126/science.abh2644. - DOI - PMC - PubMed
-
- Governo do Estado do Amazonas, Fundação de Vigilância em Saúde do Amazonas. 2021. Vacinômetro - COVID-19. https://www.fvs.am.gov.br/indicadorSalaSituacao_view/75/2. Accessed July 03, 2021.
Publication types
MeSH terms
Substances
Grants and funding
- LNCC
- E-26/202.896/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)
- VPPCB-007-FIO-18-2-30/Fundação Oswaldo Cruz (FIOCRUZ)
- PCTI-EmergeSaude/Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM)
- Rede Genô/Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

