Convalescent Plasma for Preventing Critical Illness in COVID-19: a Phase 2 Trial and Immune Profile
- PMID: 35196802
- PMCID: PMC8865433
- DOI: 10.1128/spectrum.02560-21
Convalescent Plasma for Preventing Critical Illness in COVID-19: a Phase 2 Trial and Immune Profile
Abstract
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an unprecedented event requiring frequent adaptation to changing clinical circumstances. Convalescent immune plasma (CIP) is a promising treatment that can be mobilized rapidly in a pandemic setting. We tested whether administration of SARS-CoV-2 CIP at hospital admission could reduce the rate of ICU transfer or 28-day mortality or alter levels of specific antibody responses before and after CIP infusion. In a single-arm phase II study, patients >18 years-old with respiratory symptoms with confirmed COVID-19 infection who were admitted to a non-ICU bed were administered two units of CIP within 72 h of admission. Levels of SARS-CoV-2 detected by PCR in the respiratory tract and circulating anti-SARS-CoV-2 antibody titers were sequentially measured before and after CIP transfusion. Twenty-nine patients were transfused high titer CIP and 48 contemporaneous comparable controls were identified. All classes of antibodies to the three SARS-CoV-2 target proteins were significantly increased at days 7 and 14 post-transfusion compared with baseline (P < 0.01). Anti-nucleocapsid IgA levels were reduced at day 28, suggesting that the initial rise may have been due to the contribution of CIP. The groups were well-balanced, without statistically significant differences in demographics or co-morbidities or use of remdesivir or dexamethasone. In participants transfused with CIP, the rate of ICU transfer was 13.8% compared to 27.1% for controls with a hazard ratio 0.506 (95% CI 0.165-1.554), and 28-day mortality was 6.9% compared to 10.4% for controls, hazard ratio 0.640 (95% CI 0.124-3.298). IMPORTANCE Transfusion of high-titer CIP to non-critically ill patients early after admission with COVID-19 respiratory disease was associated with significantly increased anti-SARS-CoV-2 specific antibodies (compared to baseline) and a non-significant reduction in ICU transfer and death (compared to controls). This prospective phase II trial provides a suggestion that the antiviral effects of CIP from early in the COVID-19 pandemic may delay progression to critical illness and death in specific patient populations. This study informs the optimal timing and potential population of use for CIP in COVID-19, particularly in settings without access to other interventions, or in planning for future coronavirus pandemics.
Keywords: COVID-19; SARS-CoV-2; antibodies; convalescent plasma; respiratory failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
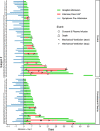
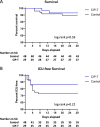
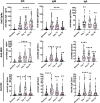
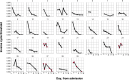
Update of
-
Convalescent plasma for preventing critical illness in COVID-19: A phase 2 trial and immune profile.medRxiv [Preprint]. 2021 Feb 19:2021.02.16.21251849. doi: 10.1101/2021.02.16.21251849. medRxiv. 2021. Update in: Microbiol Spectr. 2022 Feb 23;10(1):e0256021. doi: 10.1128/spectrum.02560-21. PMID: 33619508 Free PMC article. Updated. Preprint.
References
-
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR, Convalescent Plasma Study G. 2015. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 211:80–90. doi: 10.1093/infdis/jiu396. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

