In Vitro Antifungal Activity of Manogepix and Other Antifungal Agents against South African Candida auris Isolates from Bloodstream Infections
- PMID: 35196811
- PMCID: PMC8865435
- DOI: 10.1128/spectrum.01717-21
In Vitro Antifungal Activity of Manogepix and Other Antifungal Agents against South African Candida auris Isolates from Bloodstream Infections
Abstract
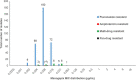
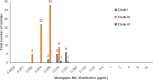
We determined the susceptibility of South African Candida auris bloodstream surveillance isolates to manogepix, a novel antifungal, and several registered antifungal agents. C. auris isolates were submitted to a reference laboratory between 2016 and 2017. Species identification was confirmed by phenotypic methods. We determined MICs for amphotericin B, anidulafungin, caspofungin, micafungin, itraconazole, posaconazole, voriconazole, fluconazole, and flucytosine using Sensititre YeastOne and manogepix using a modified Clinical and Laboratory Standards Institute broth microdilution method. Clade distribution was determined for a subset of isolates using whole-genome sequencing. Of 394 tested isolates, 357 were resistant to at least 1 antifungal class. The manogepix MIC range was 0.002 to 0.06 μg/mL for 335 isolates with fluconazole monoresistance. Nineteen isolates were resistant to both fluconazole and amphotericin B yet still had low manogepix MICs (range, 0.004 to 0.03 μg/mL). Two isolates from the same patient were panresistant but had manogepix MICs of 0.004 μg/mL and 0.008 μg/mL. Comparing MIC50 values, manogepix was >3-fold more potent than azoles, 4-fold more potent than echinocandins, and 9-fold more potent than amphotericin B. Of 84 sequenced isolates, the manogepix MIC range for 70 clade III isolates was 0.002 to 0.031 μg/mL, for 13 clade I isolates was 0.008 to 0.031 μg/mL, and for one clade IV isolate, 0.016 μg/mL. Manogepix exhibited potent activity against all isolates, including those resistant to more than one antifungal agent and in three different clades. These data support manogepix as a promising candidate for treatment of C. auris infections. IMPORTANCE Since C. auris was first detected in South Africa in 2012, health care-associated transmission events and large outbreaks have led to this pathogen accounting for more than 1 in 10 cases of candidemia. A large proportion of South African C. auris isolates are highly resistant to fluconazole but variably resistant to amphotericin B and echinocandins. There is also an emergence of pandrug-resistant C. auris isolates, limiting treatment options. Therefore, the development of new antifungal agents such as fosmanogepix or the use of new combinations of antifungal agents is imperative to the continued effective treatment of C. auris infections. Manogepix, the active moiety of fosmanogepix, has shown excellent activity against C. auris isolates. With the emergence of C. auris isolates that are pandrug-resistant in South Africa, our in vitro susceptibility data support manogepix as a promising new drug candidate for treatment of C. auris and difficult-to-treat C. auris infections.
Keywords: Candida auris; antifungal susceptibility; candidemia; fosmanogepix; manogepix; multidrug-resistant; panfungal-resistant.
Conflict of interest statement
The authors declare a conflict of interest. Amplyx Pharmaceuticals (now Pfizer) supplied the manogepix powder and was given an opportunity to review drafts of this manuscript, as per the materials transfer agreement, but the senior author (N.P.G.) made the final decisions with regard to inclusion of any suggested changes.
Figures

Similar articles
-
In Vitro Antifungal Resistance of Candida auris Isolates from Bloodstream Infections, South Africa.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0051721. doi: 10.1128/AAC.00517-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34228535 Free PMC article.
-
Manogepix (APX001A) In Vitro Activity against Candida auris: Head-to-Head Comparison of EUCAST and CLSI MICs.Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00656-20. doi: 10.1128/AAC.00656-20. Print 2020 Sep 21. Antimicrob Agents Chemother. 2020. PMID: 32660998 Free PMC article.
-
In Vitro Activity of Manogepix against Multidrug-Resistant and Panresistant Candida auris from the New York Outbreak.Antimicrob Agents Chemother. 2020 Oct 20;64(11):e01124-20. doi: 10.1128/AAC.01124-20. Print 2020 Oct 20. Antimicrob Agents Chemother. 2020. PMID: 32839219 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Candida auris and multidrug resistance: Defining the new normal.Fungal Genet Biol. 2019 Oct;131:103243. doi: 10.1016/j.fgb.2019.103243. Epub 2019 Jun 20. Fungal Genet Biol. 2019. PMID: 31228646 Free PMC article. Review.
Cited by
-
Candida auris: A Systematic Review of a Globally Emerging Fungal Pathogen in Africa.Open Forum Infect Dis. 2023 Dec 27;11(6):ofad681. doi: 10.1093/ofid/ofad681. eCollection 2024 Jun. Open Forum Infect Dis. 2023. PMID: 38887473 Free PMC article. Review.
-
TAC1b mutation in Candida auris decreases manogepix susceptibility owing to increased CDR1 expression.Antimicrob Agents Chemother. 2025 Feb 13;69(2):e0150824. doi: 10.1128/aac.01508-24. Epub 2024 Dec 18. Antimicrob Agents Chemother. 2025. PMID: 39692503 Free PMC article.
-
Population genomic analyses reveal evidence for limited recombination in the superbug Candida auris in nature.Comput Struct Biotechnol J. 2022 Jun 16;20:3030-3040. doi: 10.1016/j.csbj.2022.06.030. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35782746 Free PMC article.
-
Candida auris, a singular emergent pathogenic yeast: its resistance and new therapeutic alternatives.Eur J Clin Microbiol Infect Dis. 2022 Dec;41(12):1371-1385. doi: 10.1007/s10096-022-04497-2. Epub 2022 Oct 6. Eur J Clin Microbiol Infect Dis. 2022. PMID: 36198878 Review.
-
New Generation Modified Azole Antifungals against Multidrug-Resistant Candida auris.J Med Chem. 2025 Jul 10;68(13):14054-14071. doi: 10.1021/acs.jmedchem.5c01253. Epub 2025 Jun 23. J Med Chem. 2025. PMID: 40550788 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC). Tracking Candida auris, countries from which Candida auris cases have been reported, as of February 15, 2021. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html#historical.
-
- van Schalkwyk E, Mpembe R, Thomas J, Shuping L, Ismail H, Lowman W, Karstaedt A, Chibabhai V, Wadula J, Avenant T, Messina A, Govind C, Moodley K, Dawood H, Ramjathan P, Govender N, for GERMS-SA . 2019. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg Infect Dis 25:1698–1707. doi:10.3201/eid2509.190040. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

