CD84 is a Suppressor of T and B Cell Activation during Mycobacterium tuberculosis Pathogenesis
- PMID: 35196822
- PMCID: PMC8865571
- DOI: 10.1128/spectrum.01557-21
CD84 is a Suppressor of T and B Cell Activation during Mycobacterium tuberculosis Pathogenesis
Abstract
Interest in host-directed therapies as alternatives/adjuncts to antibiotic treatment has resurged with the increasing prevalence of antibiotic-resistant tuberculosis (TB). Immunotherapies that reinvigorate immune responses by targeting immune checkpoints like PD-1/PD-L1 have proved successful in cancer therapy. Immune cell inhibitory receptors that trigger Mycobacterium tuberculosis-specific immunosuppression, however, are unknown. Here, we show that the levels of CD84, a SLAM family receptor, increase in T and B cells in lung tissues from M. tuberculosis-infected C57BL/6 mice and in peripheral blood mononuclear cells (PBMCs) from pulmonary TB patients. M. tuberculosis challenge experiments using CD84-deficient C57BL/6 mice suggest that CD84 expression likely leads to T and B cell immunosuppression during M. tuberculosis pathogenesis and also plays an inhibitory role in B cell activation. Importantly, CD84-deficient mice showed improved M. tuberculosis clearance and longer survival than M. tuberculosis-infected wild-type (WT) mice. That CD84 is a putative M. tuberculosis infection-specific inhibitory receptor suggests it may be a suitable target for the development of TB-specific checkpoint immunotherapies. IMPORTANCE Immune checkpoint therapies, such as targeting checkpoints like PD-1/PD-L1, have proved successful in cancer therapy and can reinvigorate immune responses. The potential of this approach for treating chronic infectious diseases like TB has been recognized, but a lack of suitable immunotherapeutic targets, i.e., immune cell inhibitory receptors that trigger immunosuppression specifically during Mycobacterium tuberculosis pathogenesis, has limited the application of this strategy in the development of new TB therapies. Our focus in this study was to address this gap and search for an M. tuberculosis-specific checkpoint target. Our results suggest that CD84 is a putative inhibitory receptor that may be a suitable target for the development of TB-specific checkpoint immunotherapies.
Keywords: CD84; Mycobacterium tuberculosis; checkpoint immunotherapy; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

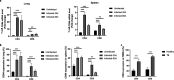


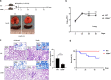
References
-
- Zumla A, Maeurer M, Chakaya J, Hoelscher M, Ntoumi F, Rustomjee R, Vilaplana C, Yeboah-Manu D, Rasolof V, Munderi P, Singh N, Aklillu E, Padayatchi N, Macete E, Kapata N, Mulenga M, Kibiki G, Mfinanga S, Nyirenda T, Maboko L, Garcia-Basteiro A, Rakotosamimanana N, Bates M, Mwaba P, Reither K, Gagneux S, Edwards S, Mfinanga E, Abdulla S, Cardona PJ, Russell JB, Gant V, Noursadeghi M, Elkington P, Bonnet M, Menendez C, Dieye TN, Diarra B, Maiga A, Aseffa A, Parida S, Wejse C, Petersen E, Kaleebu P, Oliver M, Craig G, Corrah T, Tientcheu L, Antonio M, Rao M, Host-Directed Therapies Network, et al. 2015. Towards host-directed therapies for tuberculosis. Nat Rev Drug Discov 14:511–512. doi: 10.1038/nrd4696. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

