Direct Spectroscopic Quantification of the Absorption and Scattering Properties for Single Aerosol Particles
- PMID: 35196856
- PMCID: PMC9097522
- DOI: 10.1021/acs.jpca.2c00532
Direct Spectroscopic Quantification of the Absorption and Scattering Properties for Single Aerosol Particles
Abstract
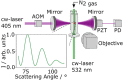
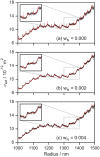
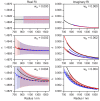
Understanding the optical properties of micrometer-scale light-absorbing aerosol particles is of paramount importance in addressing key challenges in atmospheric and physical chemistry. For example, the absorption of solar radiation by atmospheric aerosols represents one of the largest uncertainties in climate models. Moreover, reaction acceleration within the unique environments of aerosol droplets cannot be replicated in bulk solutions. The causes of these reaction rate enhancements remain controversial, but ultrasensitive spectroscopic measurements of evolving aerosol optical properties should provide new insights. We demonstrate a new approach using cavity ring-down spectroscopy that allows the first direct spectroscopic quantification of the continuously evolving absorption and scattering cross sections for single, levitated, micrometer-scale particles as their size and chromophore concentration change. For two-component droplets composed of nigrosin and 1,2,6-hexanetriol, the unprecedented sensitivity of our measurements reveals the evolving real and imaginary components of the refractive index caused by changes in concentration as 1,2,6-hexanetriol slowly evaporates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Feng Y.; Ramanathan V.; Kotamarthi V. R. Brown Carbon: A Significant Atmospheric Absorber of Solar Radiation?. Atmos. Chem. Phys. 2013, 13, 8607–8621. 10.5194/acp-13-8607-2013. - DOI
-
- IPCC , Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker T. F., Qin D., Plattner G.-K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P. M., Eds.; Cambridge University Press: Cambridge, U.K., 2013; pp 1–30.
-
- Lin G.; Penner J. E.; Flanner M. G.; Sillman S.; Xu L.; Zhou C. Radiative Forcing of Organic Aerosol in the Atmosphere and on Snow: Effects of SOA and Brown Carbon. J. Geophys. Res-Atmos. 2014, 119, 7453–7476. 10.1002/2013JD021186. - DOI

