METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer
- PMID: 35197058
- PMCID: PMC8864846
- DOI: 10.1186/s12943-021-01447-y
METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer
Abstract
Background: Continual expression of PD-L1 in tumor cells is critical for tumor immune escape and host T cell exhaustion, however, knowledge on its clinical benefits through inhibition is limited in breast cancer. N6-methyladenosine (m6A) plays a crucial role in multiple biological activities. Our study aimed to investigate the regulatory role of the m6A modification in PD-L1 expression and immune surveillance in breast cancer.
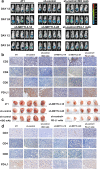
Methods: MeRIP-seq and epitranscriptomic microarray identified that PD-L1 is the downstream target of METTL3. MeRIP-qPCR, absolute quantification of m6A modification assay, and RIP-qPCR were used to examine the molecular mechanism underlying METTL3/m6A/IGF2BP3 signaling axis in PD-L1 expression. B-NDG and BALB/c mice were used to construct xenograft tumor models to verify the phenotypes upon METTL3 and IGF2BP3 silencing. In addition, breast cancer tissue microarray was used to analyze the correlation between PD-L1 and METTL3 or IGF2BP3 expression.
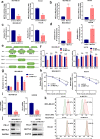
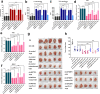
Results: We identified that PD-L1 was a downstream target of METTL3-mediated m6A modification in breast cancer cells. METTL3 knockdown significantly abolished m6A modification and reduced stabilization of PD-L1 mRNA. Additionally, METTL3-mediated PD-L1 mRNA activation was m6A-IGF2BP3-dependent. Moreover, inhibition of METTL3 or IGF2BP3 enhanced anti-tumor immunity through PD-L1-mediated T cell activation, exhaustion, and infiltration both in vitro and in vivo. PD-L1 expression was also positively correlated with METTL3 and IGF2BP3 expression in breast cancer tissues.
Conclusion: Our study suggested that METTL3 could post-transcriptionally upregulate PD-L1 expression in an m6A-IGF2BP3-dependent manner to further promote stabilization of PD-L1 mRNA, which may have important implications for new and efficient therapeutic strategies in the tumor immunotherapy.
Keywords: Breast cancer; Immune surveillance; METTL3; PD-L1; m6A.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2021. - PubMed
-
- Esteva F, Hubbard-Lucey V, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20:e175–e186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

