Clinical standards for the diagnosis, treatment and prevention of TB infection
- PMID: 35197159
- PMCID: PMC8886963
- DOI: 10.5588/ijtld.21.0753
Clinical standards for the diagnosis, treatment and prevention of TB infection
Abstract
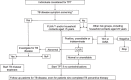
BACKGROUND: Tuberculosis (TB) preventive therapy (TPT) decreases the risk of developing TB disease and its associated morbidity and mortality. The aim of these clinical standards is to guide the assessment, management of TB infection (TBI) and implementation of TPT.METHODS: A panel of global experts in the field of TB care was identified; 41 participated in a Delphi process. A 5-point Likert scale was used to score the initial standards. After rounds of revision, the document was approved with 100% agreement.RESULTS: Eight clinical standards were defined: Standard 1, all individuals belonging to at-risk groups for TB should undergo testing for TBI; Standard 2, all individual candidates for TPT (including caregivers of children) should undergo a counselling/health education session; Standard 3, testing for TBI: timing and test of choice should be optimised; Standard 4, TB disease should be excluded prior to initiation of TPT; Standard 5, all candidates for TPT should undergo a set of baseline examinations; Standard 6, all individuals initiating TPT should receive one of the recommended regimens; Standard 7, all individuals who have started TPT should be monitored; Standard 8, a TBI screening and testing register should be kept to inform the cascade of care.CONCLUSION: This is the first consensus-based set of Clinical Standards for TBI. This document guides clinicians, programme managers and public health officers in planning and implementing adequate measures to assess and manage TBI.
CONTEXTE :: L’objectif de ces normes cliniques est d’orienter l’évaluation et la prise en charge de l’infection tuberculeuse (TBI), ainsi que la mise en place du traitement préventif antituberculeux (TPT). Le TPT réduit le risque de TB active et de morbidité et mortalité associées.
MÉTHODES :: Un panel d’experts internationaux en matière de soins antituberculeux a été identifié ; 41 experts ont participé à un processus Delphi. Une échelle de Likert à 5 points a été utilisée pour noter les normes initiales. Après plusieurs révisions, le document a été approuvé par tous les experts (100%).
RÉSULTATS :: Huit normes cliniques ont été définies : Norme 1, tous les individus des groupes à risque de TB doivent subir un test de dépistage de la TBI ; Norme 2, tous les candidats au TPT doivent prendre part à une consultation de santé informative/éducative (y compris les aidants d’enfants) ; Norme 3, test de dépistage de la TBI : le moment et le test de référence choisi doivent être optimisés; Norme 4, une TB active doit être écartée avant d’instaurer un TPT ; Norme 5, tous les candidats au TPT doivent subir différents examens initiaux ; Norme 6, tous les individus démarrant un TPT doivent recevoir l’un des protocoles recommandés ; Norme 7, tous les individus ayant démarré un TPT doivent subir des examens de contrôle ; Norme 8, un dépistage de la TBI doit être réalisé et un registre des tests doit être tenu pour orienter la cascade de soins.
CONCLUSION :: Il s’agit du premier ensemble de normes cliniques pour la TBI fondées sur un consensus. Notre objectif est d’améliorer les soins et la qualité de vie des patients en orientant les cliniciens, responsables de programme et agents de santé publique en matière de planification et de mise en place de mesures adéquates d’évaluation et de prise en charge de la TBI.
Figures
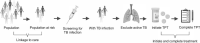

Comment in
-
The war in Ukraine and potential consequences for the TB epidemic in Europe.Int J Tuberc Lung Dis. 2022 May 1;26(5):470-471. doi: 10.5588/ijtld.22.0162. Int J Tuberc Lung Dis. 2022. PMID: 35505472 No abstract available.
-
Monitoring TB program performance.Int J Tuberc Lung Dis. 2022 Jul 1;26(7):692-693. doi: 10.5588/ijtld.22.0194. Int J Tuberc Lung Dis. 2022. PMID: 35768927 No abstract available.
-
TB in eastern Libya: a decreasing trend in recent decades.Int J Tuberc Lung Dis. 2022 Jul 1;26(7):694-695. doi: 10.5588/ijtld.22.0157. Int J Tuberc Lung Dis. 2022. PMID: 35768932 No abstract available.
-
A test of infection should not be a "standard" for guiding TB preventive therapy in at-risk populations.Int J Tuberc Lung Dis. 2022 Nov 1;26(11):1092-1093. doi: 10.5588/ijtld.22.0445. Int J Tuberc Lung Dis. 2022. PMID: 36281036 No abstract available.
-
Reply to Furin et al.: Clinical standards that are appropriate for all settings.Int J Tuberc Lung Dis. 2022 Nov 1;26(11):1093-1094. doi: 10.5588/ijtld.22.0452. Int J Tuberc Lung Dis. 2022. PMID: 36281054 No abstract available.
References
-
- World Health Organization Geneva, Switzerland: WHO; 2021. Global tuberculosis report, 2021.
-
- Cohen A, et al. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019 Sep 12;54(3):1900655. - PubMed
-
- Migliori GB, et al. Reducing tuberculosis transmission: a consensus document from the World Health Organization Regional Office for Europe. Eur Respir J. 2019;53(6):1900391. - PubMed
-
- Migliori GB, Raviglione MC. 1st ed. Switzerland: Springer, Cham; 2021. Essential tuberculosis.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

