Mathematical Models to Characterize the Absorption, Distribution, Metabolism, and Excretion of Protein Therapeutics
- PMID: 35197311
- PMCID: PMC11022906
- DOI: 10.1124/dmd.121.000460
Mathematical Models to Characterize the Absorption, Distribution, Metabolism, and Excretion of Protein Therapeutics
Abstract
Therapeutic proteins (TPs) have ranked among the most important and fastest-growing classes of drugs in the clinic, yet the development of successful TPs is often limited by unsatisfactory efficacy. Understanding pharmacokinetic (PK) characteristics of TPs is key to achieving sufficient and prolonged exposure at the site of action, which is a prerequisite for eliciting desired pharmacological effects. PK modeling represents a powerful tool to investigate factors governing in vivo disposition of TPs. In this mini-review, we discuss many state-of-the-art models that recapitulate critical processes in each of the absorption, distribution, metabolism/catabolism, and excretion pathways of TPs, which can be integrated into the physiologically-based pharmacokinetic framework. Additionally, we provide our perspectives on current opportunities and challenges for evolving the PK models to accelerate the discovery and development of safe and efficacious TPs. SIGNIFICANCE STATEMENT: This minireview provides an overview of mechanistic pharmacokinetic (PK) models developed to characterize absorption, distribution, metabolism, and elimination (ADME) properties of therapeutic proteins (TPs), which can support model-informed discovery and development of TPs. As the next-generation of TPs with diverse physicochemical properties and mechanism-of-action are being developed rapidly, there is an urgent need to better understand the determinants for the ADME of TPs and evolve existing platform PK models to facilitate successful bench-to-bedside translation of these promising drug molecules.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
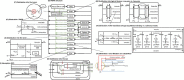
References
-
- Abuqayyas L, Balthasar JP (2012) Application of knockout mouse models to investigate the influence of FcγR on the tissue distribution and elimination of 8C2, a murine IgG1 monoclonal antibody. Int J Pharm 439:8–16. - PubMed
-
- Akilesh S, Christianson GJ, Roopenian DC, Shaw AS (2007) Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 179:4580–4588. - PubMed
-
- Arrowsmith J (2011) Trial watch: phase III and submission failures: 2007-2010. Nat Rev Drug Discov 10:87. - PubMed
-
- Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55:4611–4622. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

