From Steroid and Drug Metabolism to Glycobiology, Using Sulfotransferase Structures to Understand and Tailor Function
- PMID: 35197313
- PMCID: PMC10753775
- DOI: 10.1124/dmd.121.000478
From Steroid and Drug Metabolism to Glycobiology, Using Sulfotransferase Structures to Understand and Tailor Function
Abstract
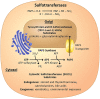
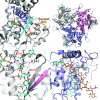
Sulfotransferases are ubiquitous enzymes that transfer a sulfo group from the universal cofactor donor 3'-phosphoadenosine 5'-phosphosulfate to a broad range of acceptor substrates. In humans, the cytosolic sulfotransferases are involved in the sulfation of endogenous compounds such as steroids, neurotransmitters, hormones, and bile acids as well as xenobiotics including drugs, toxins, and environmental chemicals. The Golgi associated membrane-bound sulfotransferases are involved in post-translational modification of macromolecules from glycosaminoglycans to proteins. The sulfation of small molecules can have profound biologic effects on the functionality of the acceptor, including activation, deactivation, or enhanced metabolism and elimination. Sulfation of macromolecules has been shown to regulate a number of physiologic and pathophysiological pathways by enhancing binding affinity to regulatory proteins or binding partners. Over the last 25 years, crystal structures of these enzymes have provided a wealth of information on the mechanisms of this process and the specificity of these enzymes. This review will focus on the general commonalities of the sulfotransferases, from enzyme structure to catalytic mechanism as well as providing examples into how structural information is being used to either design drugs that inhibit sulfotransferases or to modify the enzymes to improve drug synthesis. SIGNIFICANCE STATEMENT: This manuscript honors Dr. Masahiko Negishi's contribution to the understanding of sulfotransferase mechanism, specificity, and roles in biology by analyzing the crystal structures that have been solved over the last 25 years.
U.S. Government work not protected by U.S. copyright.
Figures
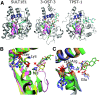

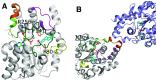

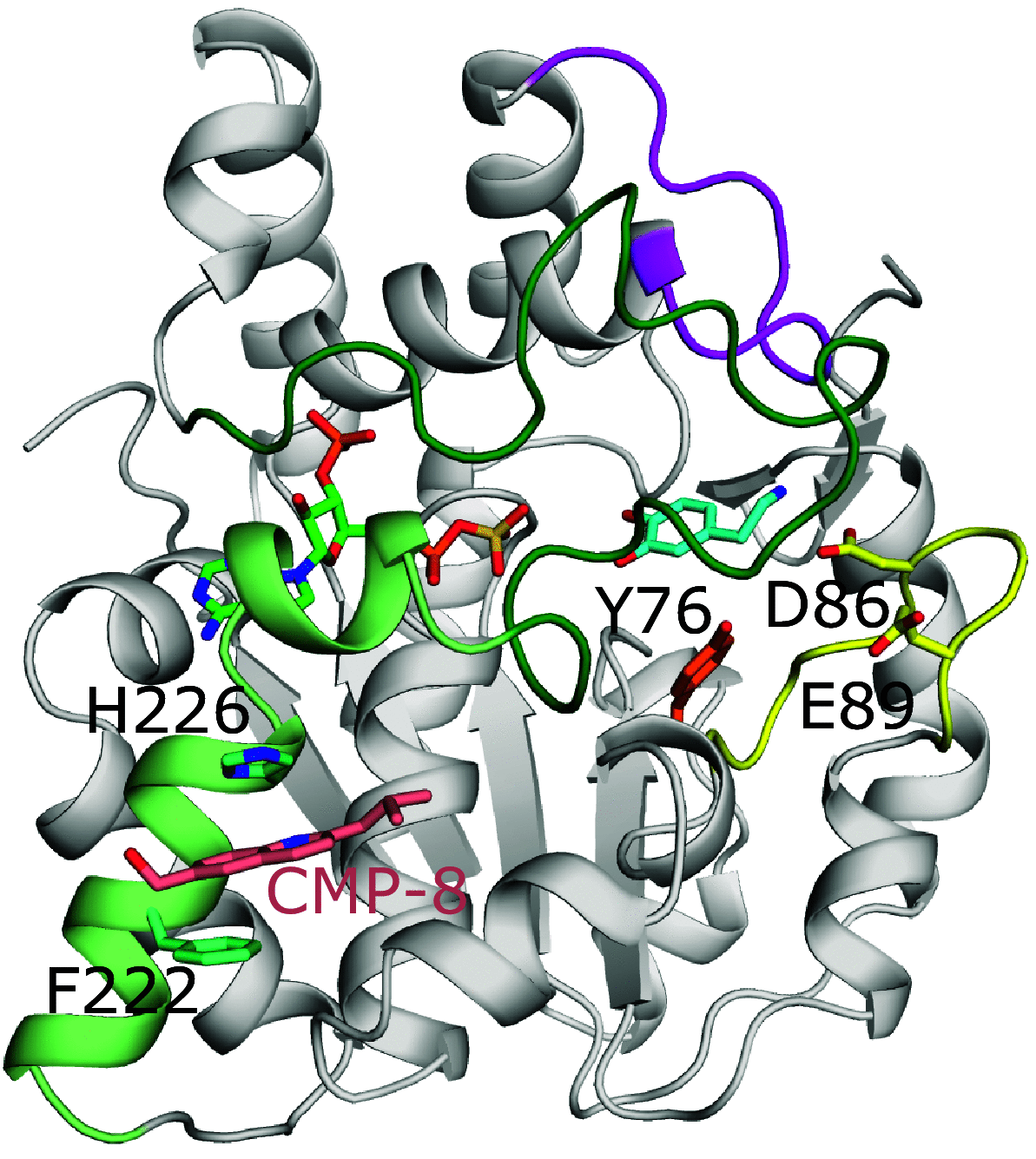
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

