Serum Protein Exposure Activates a Core Regulatory Program Driving Human Proximal Tubule Injury
- PMID: 35197326
- PMCID: PMC9063895
- DOI: 10.1681/ASN.2021060751
Serum Protein Exposure Activates a Core Regulatory Program Driving Human Proximal Tubule Injury
Abstract
Background: The kidneys efficiently filter waste products while retaining serum proteins in the circulation. However, numerous diseases compromise this barrier function, resulting in spillage of serum proteins into the urine (proteinuria). Some studies of glomerular filtration suggest that tubules may be physiologically exposed to nephrotic-range protein levels. Therefore, whether serum components can directly injure the downstream tubular portions of the kidney, which in turn can lead to inflammation and fibrosis, remains controversial.
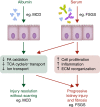
Methods: We tested the effects of serum protein exposure in human kidney tubule microphysiologic systems and with orthogonal epigenomic approaches since animal models cannot directly assess the effect of serum components on tubules.
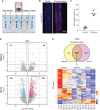
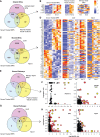
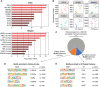
Results: Serum, but not its major protein component albumin, induced tubular injury and secretion of proinflammatory cytokines. Epigenomic comparison of serum-injured tubules and intact kidney tissue revealed canonical stress-inducible regulation of injury-induced genes. Concordant transcriptional changes in microdissected tubulointerstitium were also observed in an independent cohort of patients with proteinuric kidney disease.
Conclusions: Our results demonstrate a causal role for serum proteins in tubular injury and identify regulatory mechanisms and novel pathways for intervention.
Keywords: acute renal failure; albuminuria; blood proteins; gene expression; gene transcription; nephrotic syndrome; proteinuria; proximal tubule; renal tubular epithelial cells; transcription regulation.
Copyright © 2022 by the American Society of Nephrology.
Figures




Comment in
-
Authors' Reply: Serum Protein-induced Tubular Injury.J Am Soc Nephrol. 2022 Aug;33(8):1627-1628. doi: 10.1681/ASN.2022060657. J Am Soc Nephrol. 2022. PMID: 35906085 Free PMC article. No abstract available.
-
Serum Protein-Induced Tubular Injury.J Am Soc Nephrol. 2022 Aug;33(8):1627. doi: 10.1681/ASN.2022050568. J Am Soc Nephrol. 2022. PMID: 35906090 Free PMC article. No abstract available.
References
-
- United States Renal Data System NIoH, National Institute of Diabetes and Digestive and Kidney Diseases : 2020 USRDS Annual Data Report: epidemiology of kidney disease in the United States, 2020. Available at: https://adr.usrds.org/2020. Accessed May 1, 2021
-
- Group MoDiRDS : Effects of dietary protein restriction on the progression of moderate renal disease in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol 7: 2616–2626, 1996 - PubMed
-
- Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, et al. ; AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease : Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

