Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration
- PMID: 35197462
- PMCID: PMC8866424
- DOI: 10.1038/s41413-021-00180-y
Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration
Abstract
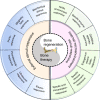
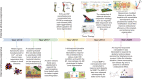
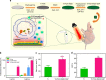
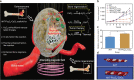
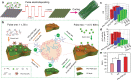
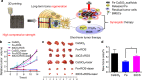
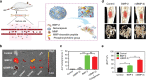
Bone defects combined with tumors, infections, or other bone diseases are challenging in clinical practice. Autologous and allogeneic grafts are two main traditional remedies, but they can cause a series of complications. To address this problem, researchers have constructed various implantable biomaterials. However, the original pathological microenvironment of bone defects, such as residual tumors, severe infection, or other bone diseases, could further affect bone regeneration. Thus, the rational design of versatile biomaterials with integrated bone therapy and regeneration functions is in great demand. Many strategies have been applied to fabricate smart stimuli-responsive materials for bone therapy and regeneration, with stimuli related to external physical triggers or endogenous disease microenvironments or involving multiple integrated strategies. Typical external physical triggers include light irradiation, electric and magnetic fields, ultrasound, and mechanical stimuli. These stimuli can transform the internal atomic packing arrangements of materials and affect cell fate, thus enhancing bone tissue therapy and regeneration. In addition to the external stimuli-responsive strategy, some specific pathological microenvironments, such as excess reactive oxygen species and mild acidity in tumors, specific pH reduction and enzymes secreted by bacteria in severe infection, and electronegative potential in bone defect sites, could be used as biochemical triggers to activate bone disease therapy and bone regeneration. Herein, we summarize and discuss the rational construction of versatile biomaterials with bone therapeutic and regenerative functions. The specific mechanisms, clinical applications, and existing limitations of the newly designed biomaterials are also clarified.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Smart stimuli-responsive strategies for titanium implant functionalization in bone regeneration and therapeutics.Mater Horiz. 2024 Jan 2;11(1):12-36. doi: 10.1039/d3mh01260c. Mater Horiz. 2024. PMID: 37818593 Review.
-
The role of smart polymeric biomaterials in bone regeneration: a review.Front Bioeng Biotechnol. 2023 Aug 17;11:1240861. doi: 10.3389/fbioe.2023.1240861. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37662432 Free PMC article. Review.
-
Stimuli-Responsive Drug Release from Smart Polymers.J Funct Biomater. 2019 Jul 31;10(3):34. doi: 10.3390/jfb10030034. J Funct Biomater. 2019. PMID: 31370252 Free PMC article. Review.
-
Smart Orthopedic Biomaterials and Implants.Curr Opin Biomed Eng. 2023 Mar;25:100439. doi: 10.1016/j.cobme.2022.100439. Epub 2022 Dec 21. Curr Opin Biomed Eng. 2023. PMID: 36642994 Free PMC article.
-
Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration.J Mater Chem B. 2019 Aug 21;7(33):5019-5037. doi: 10.1039/c9tb00847k. J Mater Chem B. 2019. PMID: 31432870 Review.
Cited by
-
Smart dental materials for antimicrobial applications.Bioact Mater. 2022 Dec 9;24:1-19. doi: 10.1016/j.bioactmat.2022.12.002. eCollection 2023 Jun. Bioact Mater. 2022. PMID: 36582351 Free PMC article.
-
Mussel-Inspired Polydopamine-Based Multilayered Coatings for Enhanced Bone Formation.Front Bioeng Biotechnol. 2022 Jul 7;10:952500. doi: 10.3389/fbioe.2022.952500. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35875492 Free PMC article. Review.
-
Advances in Regenerative Sports Medicine Research.Front Bioeng Biotechnol. 2022 May 13;10:908751. doi: 10.3389/fbioe.2022.908751. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646865 Free PMC article. Review.
-
Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials.Mater Today Bio. 2022 Jun 9;15:100318. doi: 10.1016/j.mtbio.2022.100318. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35734197 Free PMC article. Review.
-
Phenotypically complex living materials containing engineered cyanobacteria.Nat Commun. 2023 Aug 7;14(1):4742. doi: 10.1038/s41467-023-40265-2. Nat Commun. 2023. PMID: 37550278 Free PMC article.
References
-
- Sun W, et al. Injectable nano-structured silicon-containing hydroxyapatite microspheres with enhanced osteogenic differentiation and angiogenic factor expression. Ceram. Int. 2018;44:20457–20464.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

