An adjuvanted subunit SARS-CoV-2 spike protein vaccine provides protection against Covid-19 infection and transmission
- PMID: 35197485
- PMCID: PMC8866462
- DOI: 10.1038/s41541-022-00450-8
An adjuvanted subunit SARS-CoV-2 spike protein vaccine provides protection against Covid-19 infection and transmission
Abstract
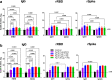
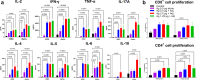
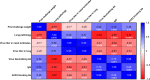
Recombinant protein approaches offer major promise for safe and effective vaccine prevention of SARS-CoV-2 infection. We developed a recombinant spike protein vaccine (called NARUVAX-C19) and characterized its ability when formulated with a nanoemulsion adjuvant to induce anti-spike antibody and T-cell responses and provide protection including against viral transmission in rodent. In mice, NARUVAX-C19 vaccine administered intramuscularly twice at 21-day interval elicited balanced Th1/Th2 humoral and T-cell responses with high titers of neutralizing antibodies against wild-type (D614G) and delta (B.1.617.2) variants. In Syrian hamsters, NARUVAX-C19 provided complete protection against wild-type (D614G) infection and prevented its transmission to naïve animals (n = 2/group) placed in the same cage as challenged animals (n = 6/group). The results contrasted with only weak protection seen with a monomeric spike receptor-binding domain (RBD) vaccine even when formulated with the same adjuvant. These encouraging results warrant the ongoing development of this COVID-19 vaccine candidate.
© 2022. The Author(s).
Conflict of interest statement
NP and LL are affiliated with Vaxine Pty Ltd which holds the rights to COVAX-19 vaccine. None of the other authors has any financial or personal interest with any organization that could inappropriately influence or bias the research activity presented in this manuscript.
Figures



References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2021. [accessed 2021 Nov]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- World Health Organization. COVID-19 vaccine tracker and landscape. [accessed 2021 Aug 03]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

