A biophysical account of multiplication by a single neuron
- PMID: 35197635
- PMCID: PMC8891015
- DOI: 10.1038/s41586-022-04428-3
A biophysical account of multiplication by a single neuron
Abstract
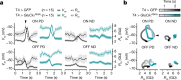
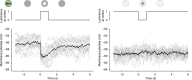
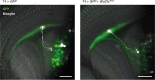
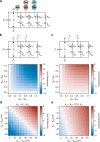
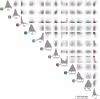
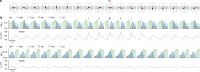
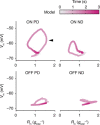
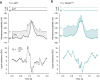
Nonlinear, multiplication-like operations carried out by individual nerve cells greatly enhance the computational power of a neural system1-3, but our understanding of their biophysical implementation is scant. Here we pursue this problem in the Drosophila melanogaster ON motion vision circuit4,5, in which we record the membrane potentials of direction-selective T4 neurons and of their columnar input elements6,7 in response to visual and pharmacological stimuli in vivo. Our electrophysiological measurements and conductance-based simulations provide evidence for a passive supralinear interaction between two distinct types of synapse on T4 dendrites. We show that this multiplication-like nonlinearity arises from the coincidence of cholinergic excitation and release from glutamatergic inhibition. The latter depends on the expression of the glutamate-gated chloride channel GluClα8,9 in T4 neurons, which sharpens the directional tuning of the cells and shapes the optomotor behaviour of the animals. Interacting pairs of shunting inhibitory and excitatory synapses have long been postulated as an analogue approximation of a multiplication, which is integral to theories of motion detection10,11, sound localization12 and sensorimotor control13.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Koch, C. & Poggio, T. Multiplying with Synapses and Neurons. In Single Neuron Computation (eds Mckenna T. et al.) 315–345 (Academic Press, 1992).
-
- Koch C, Segev I. The role of single neurons in information processing. Nat. Neurosci. 2000;3:1171–1177. - PubMed
-
- London M, Häusser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. - PubMed
-
- Joesch M, Schnell B, Raghu SV, Reiff DF, Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–304. - PubMed
-
- Maisak MS, et al. A directional tuning map of Drosophila elementary motion detectors. Nature. 2013;500:212–216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

