Prostacyclin Promotes Degenerative Pathology in a Model of Alzheimer's Disease
- PMID: 35197825
- PMCID: PMC8860182
- DOI: 10.3389/fncel.2022.769347
Prostacyclin Promotes Degenerative Pathology in a Model of Alzheimer's Disease
Abstract
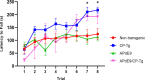
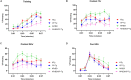
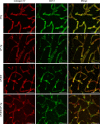
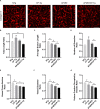
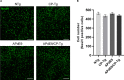
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is the most common form of dementia in aged populations. A substantial amount of data demonstrates that chronic neuroinflammation can accelerate neurodegenerative pathologies. In AD, chronic neuroinflammation results in the upregulation of cyclooxygenase and increased production of prostaglandin H2, a precursor for many vasoactive prostanoids. While it is well-established that many prostaglandins can modulate the progression of neurodegenerative disorders, the role of prostacyclin (PGI2) in the brain is poorly understood. We have conducted studies to assess the effect of elevated prostacyclin biosynthesis in a mouse model of AD. Upregulated prostacyclin expression significantly worsened multiple measures associated with amyloid-β (Aβ) disease pathologies. Mice overexpressing both Aβ and PGI2 exhibited impaired learning and memory and increased anxiety-like behavior compared with non-transgenic and PGI2 control mice. PGI2 overexpression accelerated the development of Aβ accumulation in the brain and selectively increased the production of soluble Aβ42. PGI2 damaged the microvasculature through alterations in vascular length and branching; Aβ expression exacerbated these effects. Our findings demonstrate that chronic prostacyclin expression plays a novel and unexpected role that hastens the development of the AD phenotype.
Keywords: Alzheimer’s disease; amyloid-β; neurodegeneration; neuroinflammation; prostanoid.
Copyright © 2022 Womack, Vollert, Ohia-Nwoko, Schmitt, Montazari, Beckett, Mayerich, Murphy and Eriksen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
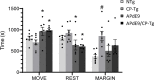
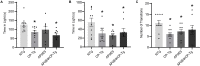



References
Grants and funding
LinkOut - more resources
Full Text Sources

