Detecting de novo Hepatic Ketogenesis Using Hyperpolarized [2-13C] Pyruvate
- PMID: 35197867
- PMCID: PMC8859440
- DOI: 10.3389/fphys.2022.832403
Detecting de novo Hepatic Ketogenesis Using Hyperpolarized [2-13C] Pyruvate
Abstract
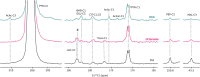
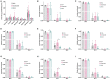
The role of ketones in metabolic health has progressed over the past two decades, moving from what was perceived as a simple byproduct of fatty acid oxidation to a central player in a multiplicity of disease states. Previous work with hyperpolarized (HP) 13C has shown that ketone production can be detected when using precursors that labeled acetyl-CoA at the C1 position, often in tissues that are not normally recognized as ketogenic. Here, we assay metabolism of HP [2-13C]pyruvate in the perfused mouse liver, a classic metabolic testbed where nutritional conditions can be precisely controlled. Livers perfused with long-chain fatty acids or the medium-chain fatty acid octanoate showed no evidence of ketogenesis in the 13C spectrum. In contrast, addition of dichloroacetate, a potent inhibitor of pyruvate dehydrogenase kinase, resulted in significant production of both acetoacetate and 3-hydroxybutyrate from the pyruvate precursor. This result indicates that ketones are readily produced from carbohydrates, but only in the case where pyruvate dehydrogenase activity is upregulated.
Keywords: dichloroacetate; hepatic metabolism; hyperpolarization; ketogenesis; octanoate metabolism.
Copyright © 2022 Ragavan, McLeod, Rushin and Merritt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


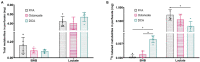
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

