Increased Fibrosis in a Mouse Model of Anti-Laminin 332 Mucous Membrane Pemphigoid Remains Unaltered by Inhibition of Aldehyde Dehydrogenase
- PMID: 35197965
- PMCID: PMC8858800
- DOI: 10.3389/fimmu.2021.812627
Increased Fibrosis in a Mouse Model of Anti-Laminin 332 Mucous Membrane Pemphigoid Remains Unaltered by Inhibition of Aldehyde Dehydrogenase
Abstract
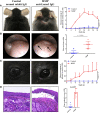
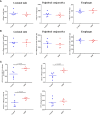
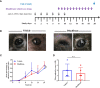
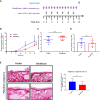
Mucous membrane pemphigoid (MMP) is an autoimmune blistering disease characterized by autoantibodies against the basal membrane zone of skin and surface-close epithelia and predominant mucosal lesions. The oral cavity and conjunctivae are most frequently affected, albeit clinical manifestations can also occur on the skin. MMP-associated lesions outside the oral cavity typically lead to scarring. Mechanisms underlying scarring are largely unknown in MMP and effective treatment options are limited. Herein, we assessed the collagen architecture in tissue samples of an antibody-transfer mouse model of anti-laminin-332 MMP. In MMP mice, increased collagen fibril density was observed in skin and conjunctival lesions compared to mice injected with normal rabbit IgG. The extracellular matrix of MMP skin samples also showed altered post-translational collagen cross-linking with increased levels of both lysine- and hydroxylysine-derived collagen crosslinks supporting the fibrotic phenotype in experimental MMP compared to control animals. In addition, we evaluated a potential anti-fibrotic therapy in experimental anti-laminin-332 MMP using disulfiram, an inhibitor of the aldehyde dehydrogenase (ALDH), which has been implicated in immune-mediated mucosal scarring. In addition, disulfiram also acts as a copper chelator that was shown to block lysyl oxidase activity, an enzyme involved in formation of collagen crosslinks. Topical use of disulfiram (300 μM in 2% [w/v] methocel) did not improve ocular lesions in experimental MMP over the 12-day treatment period in disulfiram-treated mice compared to vehicle-treated mice (n=8/group). Furthermore, C57BL6/J mice (n=8/group) were treated prophylactically with 200 mg/kg p.o. disulfiram or the solvent once daily over a period of 12 days. Systemic treatment did not show any reduction in the severity of oral and ocular lesions in MMP mice, albeit some improvement in skin lesions was observed in disulfiram- vs. vehicle-treated mice (p=0.052). No reduction in fibrosis was seen, as assessed by immunohistochemistry. Whilst blocking of ALDH failed to significantly ameliorate disease activity, our data provide new insight into fibrotic processes highlighting changes in the collagenous matrix and cross-linking patterns in IgG-mediated MMP.
Keywords: aldehyde dehydrogenase; autoimmune blistering disease; collagen; crosslinking; fibrosis; laminin 332; mouse model; mucous membrane pemphigoid.
Copyright © 2022 Patzelt, Pigors, Steenbock, Diel, Boch, Chakievska, Künzel, Busch, Fähnrich, Brinckmann and Schmidt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rashid H, Lamberts A, Borradori L, Alberti-Violetti S, Barry RJ, Caproni M, et al. . European Guidelines (S3) on Diagnosis and Management of Mucous Membrane Pemphigoid, Initiated by the European Academy of Dermatology and Venereology - Part I. J Eur Acad Dermatol Venereol (2021) 35:1750–64. doi: 10.1111/jdv.17397 - DOI - PMC - PubMed
-
- Schmidt E, Rashid H, Marzano AV, Lamberts A, Di Zenzo G, Diercks GFH, et al. . European Guidelines (S3) on Diagnosis and Management of Mucous Membrane Pemphigoid, Initiated by the European Academy of Dermatology and Venereology - Part II. J Eur Acad Dermatol Venereol (2021) 35:1926–48. doi: 10.1111/jdv.17395 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

