Convalescent Plasma Treatment in Patients with Covid-19: A Systematic Review and Meta-Analysis
- PMID: 35197981
- PMCID: PMC8859444
- DOI: 10.3389/fimmu.2022.817829
Convalescent Plasma Treatment in Patients with Covid-19: A Systematic Review and Meta-Analysis
Abstract
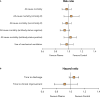
Convalescent plasma is a suggested treatment for Coronavirus disease 2019 (Covid-19), but its efficacy is uncertain. We aimed to evaluate whether the use of convalescent plasma is associated with improved clinical outcomes in patients with Covid-19.In this systematic review and meta-analysis, we searched randomized controlled trials investigating the use of convalescent plasma in patients with Covid-19 in Medline, Embase, Web of Science, Cochrane Library, and medRxiv from inception to October 17th, 2021. Two reviewers independently extracted the data. The primary efficacy outcome was all-cause mortality. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used. This study was registered with PROSPERO, CRD42021284861. Of the 8874 studies identified in the initial search, sixteen trials comprising 16 317 patients with Covid-19 were included. In the overall population, the all-cause mortality was 23.8% (2025 of 8524) with convalescent plasma and 24.4% (1903 of 7769) with standard of care (risk ratio (RR) 0.97, 95% CI 0.90-1.04) (high-certainty evidence). All-cause mortality did not differ in the subgroups of noncritically ill (21.7% [1288 of 5929] vs. 22.4% [1320 of 5882]) and critically ill (36.9% [518 of 1404] vs. 36.4% [455 of 1247]) patients with Covid-19. The use of convalescent plasma in patients who tested negative for anti-SARS-CoV-2 antibodies at baseline was not associated with significantly improved survival (RR 0.94, 95% CI 0.87-1.02). In the overall study population, initiation of mechanical ventilation (RR 0.97, 95% CI 0.88-1.07), time to clinical improvement (HR 1.09, 95% CI 0.91-1.30), and time to discharge (HR 0.95, 95% CI 0.89-1.02) were similar between the two groups. In patients with Covid-19, treatment with convalescent plasma, as compared with control, was not associated with lower all-cause mortality or improved disease progression, irrespective of disease severity and baseline antibody status.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier PROSPERO (CRD42021284861).
Keywords: SARS-CoV-2; antibodies; convalescent plasma (CP) therapy; coronavirus – COVID-19; hyperimmune globulin; passive immunization; serotherapy.
Copyright © 2022 Jorda, Kussmann, Kolenchery, Siller-Matula, Zeitlinger, Jilma and Gelbenegger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

