Characterization of Two Heterogeneous Lethal Mouse-Adapted SARS-CoV-2 Variants Recapitulating Representative Aspects of Human COVID-19
- PMID: 35197985
- PMCID: PMC8858946
- DOI: 10.3389/fimmu.2022.821664
Characterization of Two Heterogeneous Lethal Mouse-Adapted SARS-CoV-2 Variants Recapitulating Representative Aspects of Human COVID-19
Abstract
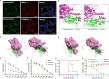
New emerging severe acute respiratory syndrome 2 (SARS-CoV-2) has caused a worldwide pandemic. Several animal models of coronavirus disease 2019 (COVID-19) have been developed and applied to antiviral research. In this study, two lethal mouse-adapted SARS-CoV-2 variants (BMA8 and C57MA14) with different virulence were generated from different hosts, which are characterized by high viral replication titers in the upper and lower respiratory tract, pulmonary pathology, cytokine storm, cellular tropism, lymphopenia, and neutrophilia. Two variants exhibit host genetics-related and age-dependent morbidity and mortality in mice, exquisitely reflecting the clinical manifestation of asymptomatic, moderate, and severe COVID-19 patients. Notably, both variants equally weaken the neutralization capacity of the serum derived from COVID-19 convalescent, but the C57MA14 variant showed a much higher virulence than the BMA8 variant in vitro. Q489H substitution in the receptor-binding domain (RBD) of BMA8 and C57MA14 variants results in the receptors of SARS-CoV-2 switching from human angiotensin-converting enzyme 2 (hACE2) to murine angiotensin-converting enzyme 2 (mACE2). Additionally, A22D and A36V mutation in E protein were first reported in our study, which potentially contributed to the virulence difference between the two variants. Of note, the protective efficacy of the novel bacterium-like particle (BLP) vaccine candidate was validated using the BMA8- or C57MA14-infected aged mouse model. The BMA8 variant- and C57MA14 variant-infected models provide a relatively inexpensive and accessible evaluation platform for assessing the efficacy of vaccines and novel therapeutic approaches. This will promote further research in the transmissibility and pathogenicity mechanisms of SARS-CoV-2.
Keywords: BLP vaccine; COVID-19; SARS-CoV-2; mouse model; mutation; pathogenesis.
Copyright © 2022 Yan, Li, Wang, Li, Liu, Wang, Qin, Su, Pei, Wang, Feng, Zhao, Yang, Xia and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Infection of wild-type mice by SARS-CoV-2 B.1.351 variant indicates a possible novel cross-species transmission route.Signal Transduct Target Ther. 2021 Dec 14;6(1):420. doi: 10.1038/s41392-021-00848-1. Signal Transduct Target Ther. 2021. PMID: 34907154 Free PMC article.
-
SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice.J Virol. 2022 Jan 12;96(1):e0151121. doi: 10.1128/JVI.01511-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668780 Free PMC article.
-
Q493K and Q498H substitutions in Spike promote adaptation of SARS-CoV-2 in mice.EBioMedicine. 2021 May;67:103381. doi: 10.1016/j.ebiom.2021.103381. Epub 2021 May 14. EBioMedicine. 2021. PMID: 33993052 Free PMC article.
-
SARS-CoV-2 Virus-Host Interaction: Currently Available Structures and Implications of Variant Emergence on Infectivity and Immune Response.Int J Mol Sci. 2021 Oct 7;22(19):10836. doi: 10.3390/ijms221910836. Int J Mol Sci. 2021. PMID: 34639178 Free PMC article. Review.
-
COVID-19 Variants and Vaccine Development.Viruses. 2024 May 10;16(5):757. doi: 10.3390/v16050757. Viruses. 2024. PMID: 38793638 Free PMC article. Review.
Cited by
-
Therapeutic equine hyperimmune antibodies with high and broad-spectrum neutralizing activity protect rodents against SARS-CoV-2 infection.Front Immunol. 2023 Feb 17;14:1066730. doi: 10.3389/fimmu.2023.1066730. eCollection 2023. Front Immunol. 2023. PMID: 36875106 Free PMC article.
-
The SARS-CoV-2 B.1.351 Variant Can Transmit in Rats But Not in Mice.Front Immunol. 2022 Apr 28;13:869809. doi: 10.3389/fimmu.2022.869809. eCollection 2022. Front Immunol. 2022. PMID: 35572504 Free PMC article.
-
Mouse-Adapted SARS-CoV-2 MA10 Strain Displays Differential Pulmonary Tropism and Accelerated Viral Replication, Neurodissemination, and Pulmonary Host Responses in K18-hACE2 Mice.mSphere. 2023 Feb 21;8(1):e0055822. doi: 10.1128/msphere.00558-22. Epub 2023 Feb 2. mSphere. 2023. PMID: 36728430 Free PMC article.
-
Animal models and SARS-CoV-2-induced pulmonary and neurological injuries.Mem Inst Oswaldo Cruz. 2023 Jan 20;117:e220239. doi: 10.1590/0074-02760220239. eCollection 2023. Mem Inst Oswaldo Cruz. 2023. PMID: 36700583 Free PMC article. Review.
-
Immunogenicity and protective potential of chimeric virus-like particles containing SARS-CoV-2 spike and H5N1 matrix 1 proteins.Front Cell Infect Microbiol. 2022 Jul 18;12:967493. doi: 10.3389/fcimb.2022.967493. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35923799 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

