Physiological Characteristics of Cotton Subtending Leaf Are Associated With Yield in Contrasting Nitrogen-Efficient Cotton Genotypes
- PMID: 35197997
- PMCID: PMC8859460
- DOI: 10.3389/fpls.2022.825116
Physiological Characteristics of Cotton Subtending Leaf Are Associated With Yield in Contrasting Nitrogen-Efficient Cotton Genotypes
Abstract
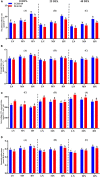
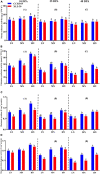
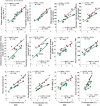
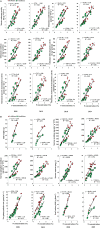
Nitrogen (N) plays an important role in various plant physiological processes, but studies on the photosynthetic efficiency and enzymatic activities in the cotton subtending leaves and their contribution to yield are still lacking. This study explored the influence of low, moderate, and high N levels on the growth, photosynthesis, carbon (C) and N metabolizing enzymes, and their contribution to yield in CCRI-69 (N-efficient) and XLZ-30 (N-inefficient). The results showed that moderate to high N levels had significantly improved growth, photosynthesis, and sucrose content of CCRI-69 as compared to XLZ-30. The seed cotton yield and lint yield of CCRI-69 were similar under moderate and high N levels but higher than XLZ-30. Similarly, moderate to high N levels improved the C/N metabolizing enzymatic activities in the subtending leaf of CCRI-69 than XLZ-30. A strong correlation was found between subtending leaf N concentration with C/N metabolizing enzymes, photosynthesis, sucrose contents, boll weight, and seed cotton yield of N-efficient cotton genotype. These findings suggest that subtending leaf N concentration regulates the enzymatic activities and has a key role in improving the yield. These parameters may be considered for breeding N-efficient cotton genotypes, which might help to reduce fertilizer loss and improve crop productivity.
Keywords: cotton; cotton subtending leaf; enzymatic activities; nitrogen; photosynthesis; yield.
Copyright © 2022 Iqbal, Jing, Qiang, Xiangru, Huiping, Hengheng, Nianchang, Xiling and Meizhen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Genotypic variation in carbon and nitrogen metabolism in the cotton subtending leaves and seed cotton yield under various nitrogen levels.J Sci Food Agric. 2023 Mar 30;103(5):2602-2617. doi: 10.1002/jsfa.12412. Epub 2023 Jan 14. J Sci Food Agric. 2023. PMID: 36571565
-
Photosynthetic characteristics of the subtending leaf of cotton boll at different fruiting branch nodes and their relationships with lint yield and fiber quality.Front Plant Sci. 2015 Sep 17;6:747. doi: 10.3389/fpls.2015.00747. eCollection 2015. Front Plant Sci. 2015. PMID: 26442060 Free PMC article.
-
Changes in carbohydrate distribution in cotton photosynthetic organs and increase in boll weight reduce yield loss under high temperature.J Exp Bot. 2024 Jun 7;75(11):3483-3499. doi: 10.1093/jxb/erae122. J Exp Bot. 2024. PMID: 38483180
-
Dissecting Source-Sink Relationship of Subtending Leaf for Yield and Fiber Quality Attributes in Upland Cotton (Gossypium hirsutum L.).Plants (Basel). 2021 Jun 4;10(6):1147. doi: 10.3390/plants10061147. Plants (Basel). 2021. PMID: 34199872 Free PMC article.
-
The physiological response of photosynthesis to nitrogen deficiency.Plant Physiol Biochem. 2021 Jan;158:76-82. doi: 10.1016/j.plaphy.2020.11.019. Epub 2020 Nov 17. Plant Physiol Biochem. 2021. PMID: 33296848 Review.
Cited by
-
Phosphorus and carbohydrate metabolism contributes to low phosphorus tolerance in cotton.BMC Plant Biol. 2023 Feb 16;23(1):97. doi: 10.1186/s12870-023-04100-6. BMC Plant Biol. 2023. PMID: 36792994 Free PMC article.
-
Physiological and Structural Changes in Leaves of Platycrater arguta Seedlings Exposed to Increasing Light Intensities.Plants (Basel). 2024 Apr 30;13(9):1263. doi: 10.3390/plants13091263. Plants (Basel). 2024. PMID: 38732478 Free PMC article.
-
Differential responses of contrasting low phosphorus tolerant cotton genotypes under low phosphorus and drought stress.BMC Plant Biol. 2023 Mar 30;23(1):168. doi: 10.1186/s12870-023-04171-5. BMC Plant Biol. 2023. PMID: 36997867 Free PMC article.
-
Phosphorus Supplementation Enhances Growth and Antioxidant Defense Against Cadmium Stress in Cotton.Antioxidants (Basel). 2025 Jun 5;14(6):686. doi: 10.3390/antiox14060686. Antioxidants (Basel). 2025. PMID: 40563320 Free PMC article.
References
-
- Ali A., Sivakami S., Raghuram N. (2007). Effect of nitrate, nitrite, ammonium, glutamate, glutamine and 2-oxoglutarate on the RNA levels and enzyme activities of nitrate reductase and nitrite reductase in rice. Physiol. Mol. Biol. Plants 13 17–25.
-
- Ali S., Hafeez A., Ma X., Tung S. A., Liu A., Shah A. N., et al. (2018). Potassium relative ratio to nitrogen considerably favors carbon metabolism in late-planted cotton at high planting density. F. Crop. Res. 223 48–56. 10.1016/j.fcr.2018.04.005 - DOI
-
- Bondada B. R., Oosterhuis D. M. (2001). Canopy photosynthesis, specific leaf weight, and yield components of cotton under varying nitrogen supply. J. Plant Nutr. 24 469–477. 10.1081/pln-100104973 - DOI
-
- Cao X., Jia J. B., Li H., Li M. C., Luo J., Liang Z. S., et al. (2012). Photosynthesis, water use efficiency and stable carbon isotope composition are associated with anatomical properties of leaf and xylem in six poplar species. Plant Biol. 14 612–620. 10.1111/j.1438-8677.2011.00531.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

