Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury
- PMID: 35198061
- PMCID: PMC8825582
- DOI: 10.7150/thno.64963
Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury
Abstract
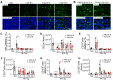
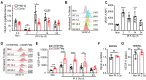
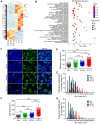
Background: Irradiation disrupts the vascular niche where hematopoietic stem cells (HSCs) reside, causing delayed hematopoietic reconstruction. The subsequent recovery of sinusoidal vessels is key to vascular niche regeneration and a prerequisite for hematopoietic reconstruction. We hypothesize that resident bone marrow macrophages (BM-Mφs) are responsible for repairing the HSC niche upon irradiation injury. Methods: We examined the survival and activation of BM-Mφs in C57BL/6 mice upon total body irradiation. After BM-Mφ depletion via injected clodronate-containing liposomes and irradiation injury, hematopoietic reconstruction and sinusoidal vascular regeneration were assessed with immunofluorescence and flow cytometry. Then enzyme-linked immunosorbent assay (ELISA) and flow cytometry were performed to analyze the contribution of VEGF-A released by BM-Mφs to the vascular restructuring of the HSC niche. VEGF-A-mediated signal transduction was assessed with transcriptome sequencing, flow cytometry, and pharmacology (agonists and antagonists) to determine the molecular mechanisms of Piezo1-mediated responses to structural changes in the HSC niche. Results: The depletion of BM-Mφs aggravated the post-irradiation injury, delaying the recovery of sinusoidal endothelial cells and HSCs. A fraction of the BM-Mφ population persisted after irradiation, with residual BM-Mφ exhibiting an activated M2-like phenotype. The expression of VEGF-A, which is essential for sinusoidal regeneration, was upregulated in BM-Mφs post-irradiation, especially CD206+ BM-Mφs. The expression of mechanosensory ion channel Piezo1, a response to mechanical environmental changes induced by bone marrow ablation, was upregulated in BM-Mφs, especially CD206+ BM-Mφs. Piezo1 upregulation was mediated by the effects of irradiation, the activation of Piezo1 itself, and the M2-like polarization induced by the phagocytosis of apoptotic cells. Piezo1 activation was associated with increased expression of VEGF-A and increased accumulation of NFATC1, NFATC2, and HIF-1α. The Piezo1-mediated upregulation in VEGF-A was suppressed by inhibiting the calcineurin/NFAT/HIF-1α signaling pathway. Conclusion: These findings reveal that BM-Mφs play a critical role in promoting vascular niche regeneration by sensing and responding to structural changes after irradiation injury, offering a potential target for therapeutic efforts to enhance hematopoietic reconstruction.
Keywords: Piezo1; hematopoietic reconstitution; irradiation; macrophages; sinusoidal regeneration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Dholaria B, Labopin M, Angelucci E, Ciceri F, Diez-Martin JL, Bruno B. et al. Impact of total body irradiation- vs chemotherapy-based myeloablative conditioning on outcomes of haploidentical hematopoietic cell transplantation for acute myelogenous leukemia. Am J Hematol. 2020;95(10):1200–1208. - PubMed
-
- Umeda K, Yabe H, Kato K, Imai K, Kobayashi M, Takahashi Y. et al. Impact of low-dose irradiation and in vivo T-cell depletion on hematopoietic stem cell transplantation for non-malignant diseases using fludarabine-based reduced-intensity conditioning. Bone Marrow Transplant. 2019;54(8):1227–1236. - PubMed
-
- Guerra JA, Dhall G, Marachelian A, Castillo E, Malvar J, Wong K. et al. Marrow-ablative chemotherapy followed by tandem autologous hematopoietic cell transplantation in pediatric patients with malignant brain tumors. Bone Marrow Transplant. 2017;52(11):1543–1548. - PubMed
-
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F. et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–4828. - PubMed

