Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment
- PMID: 35198062
- PMCID: PMC8825579
- DOI: 10.7150/thno.68304
Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment
Abstract
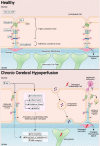
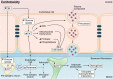
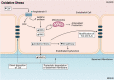
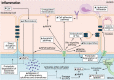
The prevalence of cerebrovascular disease increases with age, placing the elderly at a greater lifetime risk for dementia. Vascular cognitive impairment (VCI) encompasses a spectrum of cognitive deficits from mild cognitive impairment to dementia. VCI and its most severe form, vascular dementia (VaD), is becoming a major public health concern worldwide. As growing efforts are being taken to understand VCI and VaD in animal models and humans, the pathogenesis of the disease is being actively explored. It is postulated that chronic cerebral hypoperfusion (CCH) is a major cause of VCI. CCH activates a molecular and cellular injury cascade that leads to breakdown of the blood brain barrier (BBB) and neurodegeneration. The BBB tightly regulates the movement of substances between the blood and the brain, thereby regulating the microenvironment within the brain parenchyma. Here we illustrate how BBB damage is causal in the pathogenesis of VCI through the increased activation of pathways related to excitotoxicity, oxidative stress, inflammation and matrix metalloproteinases that lead to downstream perivascular damage, leukocyte infiltration and white matter changes in the brain. Thus, CCH-induced BBB damage may initiate and contribute to a vicious cycle, resulting in progressive neuropathological changes of VCI in the brain. This review outlines the molecular and cellular mechanisms that govern BBB breakdown during CCH and highlights the clinical evidence in identifying at-risk VCI patients.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo J-M, López-Pousa S. et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–7. - PubMed
-
- van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH. et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4:1–16. - PubMed
-
- Akinyemi R, Mukaetova-Ladinska E, Attems J, Ihara M, Kalaria R. Vascular Risk Factors and Neurodegeneration in Ageing Related Dementias: Alzheimer's Disease and Vascular Dementia. Curr Alzheimer Res. 2013;10:642–53. - PubMed
-
- Gyanwali B, Shaik M, Tan B, Venketasubramanian N, Chen C, Hilal S. Risk Factors for and Clinical Relevance of Incident and Progression of Cerebral Small Vessel Disease Markers in an Asian Memory Clinic Population. J Alzheimers Dis. 2019;67:1209–19. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

