Preventive effect of biodegradable stents on biliary stricture and fibrosis after biliary anastomosis in a porcine model
- PMID: 35198512
- PMCID: PMC8831087
- DOI: 10.4174/astr.2022.102.2.90
Preventive effect of biodegradable stents on biliary stricture and fibrosis after biliary anastomosis in a porcine model
Abstract
Purpose: The current drain tubes for preventing surgically biliary anastomotic stricture are not naturally and easily removed. If a drain tube using biodegradable material is easily available and the degradation time of the tube is well controlled, surgical anastomotic stricture and fibrosis could be prevented. The aim of this animal study was to evaluate the preventive effect of novel biodegradable stents (BS) on biliary stricture and fibrosis after duct-to-duct (DD) biliary anastomosis.
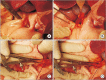
Methods: Ten mini-pigs were allocated to the control group (n = 5) and or the stent group (n = 5). The common bile duct was exposed through surgical laparotomy and then resected transversely. In the stent group, a 4-mm or 6-mm polydioxanone/magnesium sheath-core BS was inserted according to the width of the bile duct, followed by DD biliary anastomosis. In the control group, DD biliary anastomosis was performed without BS insertion.
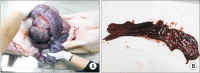
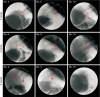
Results: In the stent group, stents were observed without deformity for up to 4 weeks in all animals. Eight weeks later, histopathologic examination revealed that the common bile duct of the anastomosis site was relatively narrower in circumference in the control group compared to the stent group. The degree of fibrosis in the control group was more marked than in the stent group (3.84 mm vs. 0.68 mm, respectively; P < 0.05).
Conclusion: Our study showed that novel BS maintained their original shape and radial force for an adequate time and then disappeared without adverse events. The BS could prevent postoperative complications and strictures after DD biliary anastomosis.
Keywords: Anastomosis; Bile ducts; Biliary stents; Biodegradable; Stents.
Copyright © 2022, the Korean Surgical Society.
Conflict of interest statement
Conflict of Interest: Kyu Seok Kim, Jong Pil Moon, Sehwan Park, and Jinkyung Jeon are researchers at the Interventional Research Center of M.I.Tech Co., Ltd. which provided the prototype biodegradable stents used in our experiment. The other authors have no conflicts of interest to disclose.
Figures




References
-
- Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842–1848. - PubMed
-
- Dulundu E, Sugawara Y, Sano K, Kishi Y, Akamatsu N, Kaneko J, et al. Duct-to-duct biliary reconstruction in adult living-donor liver transplantation. Transplantation. 2004;78:574–579. - PubMed
-
- Yazumi S, Yoshimoto T, Hisatsune H, Hasegawa K, Kida M, Tada S, et al. Endoscopic treatment of biliary complications after right-lobe living-donor liver transplantation with duct-to-duct biliary anastomosis. J Hepatobiliary Pancreat Surg. 2006;13:502–510. - PubMed
-
- Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006;101:2230–2236. - PubMed
-
- Chok KS, Lo CM. Biliary complications in right lobe living donor liver transplantation. Hepatol Int. 2016;10:553–558. - PubMed
LinkOut - more resources
Full Text Sources

