Development of Metal-Organic Framework-Based Dual Antibody Nanoparticles for the Highly Specific Capture and Gradual Release of Circulating Tumor Cells
- PMID: 35198544
- PMCID: PMC8859420
- DOI: 10.3389/fbioe.2022.806238
Development of Metal-Organic Framework-Based Dual Antibody Nanoparticles for the Highly Specific Capture and Gradual Release of Circulating Tumor Cells
Abstract
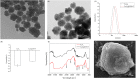
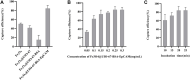
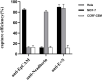
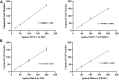
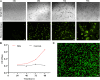
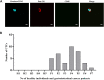
Circulating tumor cells (CTCs) have been well-established as promising biomarkers that can be leveraged to gauge the prognosis of patients with cancers and to guide patient treatment efforts. Although the scarcity of CTCs within peripheral circulation and the associated phenotypic changes that they exhibit owing to the epithelial-mesenchymal transition (EMT) process make the reliable isolation of these cells very challenging. Recently, several studies have discussed platforms capable of mediating the efficient and sensitive isolation of CTCs, but these approaches are nonetheless subject to certain limitations that preclude their clinical application. For example, these platforms are poorly-suited to minimizing damage in the context of cellular capture and release or the in vitro culture of captured cells for subsequent molecular analyses, which would better enable clinicians to select appropriate precision treatments on an individualized basis. In this study, we report the layer-by-layer assembly approach to synthesize a novel composite nanomaterial consisting of modified zirconium-based metal-organic-frameworks (MOFs) on the surface of magnetic beads with dual antibody surface modifications capable of capturing CTCs without being hampered by the state of cellular EMT process. Our analyses indicated that these dual antibody-modified nanomaterials exhibited greater capture efficiency than that observed for single antibody. Importantly, captured cells can be gradually released following capture and undergo subsequent in vitro proliferation following water molecule-induced MOF structural collapse. This release mechanism, which does not require operator intervention, may be effective as a means of minimizing damage and preserving cellular viability such that cells can be more reliably utilized for downstream molecular analyses and associated treatment planning. To further confirm the potential clinical applicability of the developed nanomaterial, it was successfully utilized for capturing CTCs from peripheral blood samples collected from cases diagnosed with gastrointestinal tumors.
Keywords: cell release; circulating tumor cells; isolation; layer-by-layer assembly method; metal organic frameworks.
Copyright © 2022 Hu, Li, Wang, Ding, Pei, Wang, Xu and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chen R., Zhang J., Chelora J., Xiong Y., Kershaw S. V., Li K. F., et al. (2017). Ruthenium(II) Complex Incorporated UiO-67 Metal-Organic Framework Nanoparticles for Enhanced Two-Photon Fluorescence Imaging and Photodynamic Cancer Therapy. ACS Appl. Mater. Inter. 9 (7), 5699–5708. 10.1021/acsami.6b12469 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

