Biogenesis and Breakdown of Lipid Droplets in Pathological Conditions
- PMID: 35198567
- PMCID: PMC8860030
- DOI: 10.3389/fcell.2021.826248
Biogenesis and Breakdown of Lipid Droplets in Pathological Conditions
Abstract
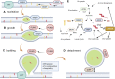
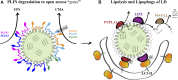
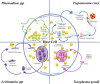
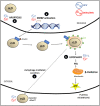
Lipid droplets (LD) have long been considered as mere fat drops; however, LD have lately been revealed to be ubiquitous, dynamic and to be present in diverse organelles in which they have a wide range of key functions. Although incompletely understood, the biogenesis of eukaryotic LD initiates with the synthesis of neutral lipids (NL) by enzymes located in the endoplasmic reticulum (ER). The accumulation of NL leads to their segregation into nanometric nuclei which then grow into lenses between the ER leaflets as they are further filled with NL. The lipid composition and interfacial tensions of both ER and the lenses modulate their shape which, together with specific ER proteins, determine the proneness of LD to bud from the ER toward the cytoplasm. The most important function of LD is the buffering of energy. But far beyond this, LD are actively integrated into physiological processes, such as lipid metabolism, control of protein homeostasis, sequestration of toxic lipid metabolic intermediates, protection from stress, and proliferation of tumours. Besides, LD may serve as platforms for pathogen replication and defense. To accomplish these functions, from biogenesis to breakdown, eukaryotic LD have developed mechanisms to travel within the cytoplasm and to establish contact with other organelles. When nutrient deprivation occurs, LD undergo breakdown (lipolysis), which begins with the LD-associated members of the perilipins family PLIN2 and PLIN3 chaperone-mediated autophagy degradation (CMA), a specific type of autophagy that selectively degrades a subset of cytosolic proteins in lysosomes. Indeed, PLINs CMA degradation is a prerequisite for further true lipolysis, which occurs via cytosolic lipases or by lysosome luminal lipases when autophagosomes engulf portions of LD and target them to lysosomes. LD play a crucial role in several pathophysiological processes. Increased accumulation of LD in non-adipose cells is commonly observed in numerous infectious diseases caused by intracellular pathogens including viral, bacterial, and parasite infections, and is gradually recognized as a prominent characteristic in a variety of cancers. This review discusses current evidence related to the modulation of LD biogenesis and breakdown caused by intracellular pathogens and cancer.
Keywords: LD biogenesis; LD breakdown; cancer; lipid droplet (LD); protozoans; viral infection.
Copyright © 2022 Fader Kaiser, Romano, Vanrell, Pocognoni, Jacob, Caruso and Delgui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

