The EHA Research Roadmap: Hematopoietic Stem Cell Gene Therapy
- PMID: 35198856
- PMCID: PMC8855740
- DOI: 10.1097/HS9.0000000000000671
The EHA Research Roadmap: Hematopoietic Stem Cell Gene Therapy
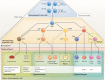
Figures
References
-
- Ferrari G, Thrasher AJ, Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat Rev Genet. 2021;22:216–234. - PubMed
-
- Porteus MH. A new class of medicines through DNA editing. N Engl J Med. 2019;380:947–959. - PubMed
-
- Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–844. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
