SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated bymultiple physiologically relevant NAD metabolites
- PMID: 35198877
- PMCID: PMC8844822
- DOI: 10.1016/j.isci.2022.103812
SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated bymultiple physiologically relevant NAD metabolites
Abstract
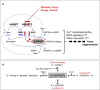
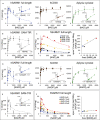
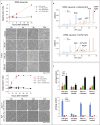
SARM1 is an NAD(P) glycohydrolase and TLR adapter with an essential, prodegenerative role in programmed axon death (Wallerian degeneration). Like other NAD(P)ases, it catalyzes multiple reactions that need to be fully investigated. Here, we compare these multiple activities for recombinant human SARM1, human CD38, and Aplysia californica ADP ribosyl cyclase. SARM1 has the highest transglycosidation (base exchange) activity at neutral pH and with some bases this dominates NAD(P) hydrolysis and cyclization. All SARM1 activities, including base exchange at neutral pH, are activated by an increased NMN:NAD ratio, at physiological levels of both metabolites. SARM1 base exchange occurs also in DRG neurons and is thus a very likely physiological source of calcium-mobilizing agent NaADP. Finally, we identify regulation by free pyridines, NADP, and nicotinic acid riboside (NaR) on SARM1, all of therapeutic interest. Understanding which specific SARM1 function(s) is responsible for axon degeneration is essential for its targeting in disease.
Keywords: Biological sciences; Molecular physiology; Neuroscience.
© 2022 The Authors.
Conflict of interest statement
Funding for academic research from AstraZeneca and M.P.C. is a consultant for Nura Bio.
Figures




References
-
- Belenky P., Christensen K.C., Gazzaniga F., Pletnev A.A., Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J. Biol. Chem. 2009;284:158–164. doi: 10.1074/jbc.M807976200. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

