Serological evaluation of the effectiveness of reactive focal mass drug administration and reactive vector control to reduce malaria transmission in Zambezi Region, Namibia: Results from a secondary analysis of a cluster randomised trial
- PMID: 35198913
- PMCID: PMC8851292
- DOI: 10.1016/j.eclinm.2022.101272
Serological evaluation of the effectiveness of reactive focal mass drug administration and reactive vector control to reduce malaria transmission in Zambezi Region, Namibia: Results from a secondary analysis of a cluster randomised trial
Abstract
Background: Due to challenges in measuring changes in malaria at low transmission, serology is increasingly being used to complement clinical and parasitological surveillance. Longitudinal studies have shown that serological markers, such as Etramp5.Ag1, can reflect spatio-temporal differences in malaria transmission. However, these markers have yet to be used as endpoints in intervention trials.
Methods: Based on data from a 2017 cluster randomised trial conducted in Zambezi Region, Namibia, evaluating the effectiveness of reactive focal mass drug administration (rfMDA) and reactive vector control (RAVC), this study conducted a secondary analysis comparing antibody responses between intervention arms as trial endpoints. Antibody responses were measured on a multiplex immunoassay, using a panel of eight serological markers of Plasmodium falciparum infection - Etramp5.Ag1, GEXP18, HSP40.Ag1, Rh2.2030, EBA175, PfMSP119, PfAMA1, and PfGLURP.R2.
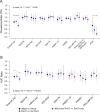
Findings: Reductions in sero-prevalence to antigens Etramp.Ag1, PfMSP119, Rh2.2030, and PfAMA1 were observed in study arms combining rfMDA and RAVC, but only effects for Etramp5.Ag1 were statistically significant. Etramp5.Ag1 sero-prevalence was significantly lower in all intervention arms. Compared to the reference arms, adjusted prevalence ratio (aPR) for Etramp5.Ag1 was 0.78 (95%CI 0.65 - 0.91, p = 0.0007) in the rfMDA arms and 0.79 (95%CI 0.67 - 0.92, p = 0.001) in the RAVC arms. For the combined rfMDA plus RAVC intervention, aPR was 0.59 (95%CI 0.46 - 0.76, p < 0.0001). Significant reductions were also observed based on continuous antibody responses. Sero-prevalence as an endpoint was found to achieve higher study power (99.9% power to detect a 50% reduction in prevalence) compared to quantitative polymerase chain reaction (qPCR) prevalence (72.9% power to detect a 50% reduction in prevalence).
Interpretation: While the observed relative reduction in qPCR prevalence in the study was greater than serology, the use of serological endpoints to evaluate trial outcomes measured effect size with improved precision and study power. Serology has clear application in cluster randomised trials, particularly in settings where measuring clinical incidence or infection is less reliable due to seasonal fluctuations, limitations in health care seeking, or incomplete testing and reporting.
Funding: This study was supported by Novartis Foundation (A122666), the Bill & Melinda Gates Foundation (OPP1160129), and the Horchow Family Fund (5,300,375,400).
Keywords: Cluster randomised trials; Malaria; Serology.
© 2022 The Author(s).
Conflict of interest statement
MSH declares research grants to her institution from Novartis Foundation, Bill & Melinda Gates Foundation and Horchow Foundation to conduct the cluster randomised trial. IK declares research grants to Wits Health Consortium from the Bill & Melinda Gates Foundation to conduct the cluster randomised trial and to support field visits and attendance at project meetings and scientific conferences related to the trial. DM declares research grants to the University of Namibia from Novartis Foundation and the Bill & Melinda Gates Foundation via a subaward from UCSF. JLS declares receiving salary from the Bill & Melinda Gates Foundation grant that co-funded the study.
Figures


References
-
- Dron L., et al. The role and challenges of cluster randomised trials for global health. Lancet Glob Heal. 2021;9:e701–e710. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

