Vapor construction and modification of stem cell-laden multicomponent scaffolds for regenerative therapeutics
- PMID: 35198961
- PMCID: PMC8850674
- DOI: 10.1016/j.mtbio.2022.100213
Vapor construction and modification of stem cell-laden multicomponent scaffolds for regenerative therapeutics
Abstract
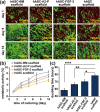
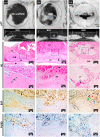
Tissue engineering based on the combined use of isolated cells, scaffolds, and growth factors is widely used; however, the manufacture of cell-preloaded scaffolds faces challenges. Herein, we fabricated a multicomponent scaffold with multiple component accommodations, including bioactive molecules (BMs), such as fibroblast growth factor-2 (FGF-2) and l-ascorbic acid 2-phosphate (A2-P), and living cells of human adipose-derived stem cells (hASCs), within one scaffold construct. We report an innovative fabrication process based on vapor-phased construction using iced templates for vapor sublimation. Simultaneously, the vaporized water molecules were replaced by vapor deposition of poly-p-xylylene (PPX, USP Class VI, highly compatible polymer, FDA-approved records), forming a three-dimensional and porous scaffold matrix. More importantly, a multicomponent modification was achieved based on using nonvolatile solutes, including bioactive molecules of FGF-2 and A2-P, and living cells of hASCs, to prepare iced templates for sublimation. Additionally, the fabrication and construction resulted in a multicomponent scaffold product comprising the devised molecules, cells, and vapor-polymerized poly-p-xylylene as the scaffold matrix. The clean and dry fabrication process did not require catalysts, initiators or plasticizers, and potentially harmful solvents, and the scaffold products were produced in simple steps within hours of the processing time. Cell viability analysis showed a high survival rate (approximately 86.4%) for the accommodated hASCs in the fabricated scaffold product, and a surprising multilineage differentiation potential of hASCs was highly upregulated because of synergistic guidance by the same accommodated FGF-2 and A2-P components. Proliferation and self-renewal activities were also demonstrated with enhancement of the multicomponent scaffold product. Finally, in vivo calvarial defect studies further revealed that the constructed scaffolds provided blood vessels to grow into the bone defect areas with enhancement, and the induced conduction of osteoblast growth also promoted bone healing toward osseointegration. The reported scaffold construction technology represents a prospective tissue engineering scaffold product to enable accommodable and customizable versatility to control the distribution and composition of loading delicate BMs and living hASCs in one scaffold construct and demonstrates unlimited applications in tissue engineering repair and regenerative medicine applications.
Keywords: Growth factor; Human adipose tissue-derived stem cell; Multifunctional scaffold; Regenerative medicine; Vapor construction.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
LinkOut - more resources
Full Text Sources

